Difference between revisions of "Part:BBa K1689004"
| (13 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1689004 short</partinfo> | <partinfo>BBa_K1689004 short</partinfo> | ||
| − | + | N-luc398-FRB fusion protein ORF | |
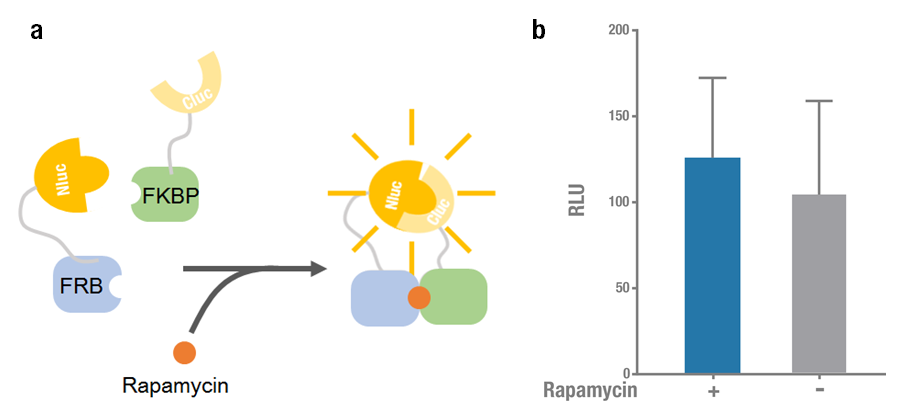
| − | Firefly (Photinus pyralis) luciferase can be split to N terminal ( | + | Firefly (<I>Photinus pyralis</I>) luciferase can be split to N-terminal (N-luc) and C-terminal (C-luc) fragments and each of them is inactive. When they two reassembled non-covalently, the enzymatic activity would be reconstituted and the recovered luciferase is able to oxidize luciferin and produce detectable bioluminescence. Currently there are different combinations of split fragments, among which N-luc416/ C-luc398 and N-luc398/ C-luc394 are widely used[1]. |
| + | |||
| + | Rapamycin-binding domain (FRB) of human mTOR (mammalian Target of Rapamycin) binds with high affinity to FK-506-binding protein 12 (FKBP). Previously Raik Gruenberg had already designed the part [https://parts.igem.org/Part:BBa_J18926 BBa_J18926], containing the coding sequence of FRB. Rapamycin is able to induce the dimerization to form a FRB-rapamycin-FKBP complex[2]. This protein-protein interaction can be visualized by split luciferase[3]. FRB and FKBP are fused to N-luc and C-luc respectively, and adding rapamycin can induce the approaching and reconstitution of split luciferase (Figure 1a). | ||
| + | |||
| + | 2015 Peking iGEM improved the previous part [https://parts.igem.org/Part:BBa_J18926 BBa_J18926], they fused N-luc398 to N terminus of FRB (N-luc398-FRB, BBa_K1689004) and combined it with FKBP-Cluc394 [https://parts.igem.org/wiki/index.php?title=Part:BBa_K1689006 (BBa_K1689006)] to validate the functional reconstitution of split luciferase. However, compared with N-luc416/ C-luc398, the bioluminescence intensity didn't increase significantly after rapamycin was added (Figure 1). Therefore we discarded them and chose N-luc416/ C-luc398 as our split luciferase in the project (See [https://parts.igem.org/wiki/index.php?title=Part:BBa_K1689003 BBa_K1689003] or [https://parts.igem.org/wiki/index.php?title=Part:BBa_K1689005 BBa_K1689005]). | ||
| − | |||
| − | |||
| Line 17: | Line 19: | ||
| − | '''Figure 1. Rapamycin-induced N-luc-FRB/FKBP-C-luc complementation. (a) The working mechanism of rapamycin induced dimerization. The interacting protein partners (FRB & FKBP) get closer and dimerize soon after rapamycin is added (40nM) [ | + | '''Figure 1. Rapamycin-induced N-luc-FRB/ FKBP-C-luc complementation. (a) The working mechanism of rapamycin induced dimerization. The interacting protein partners (FRB & FKBP) get closer and dimerize soon after rapamycin is added (40nM) [3], thus to reconstitute the enzymatic activity of luciferase. (b) The experimental data. Error bars denote s.d.; n=3. ''' |
| Line 28: | Line 30: | ||
Nat. Med. 1996. 2:1028–1032. | Nat. Med. 1996. 2:1028–1032. | ||
| − | 3. Ramasamy Paulmurugan, Sanjiv S. Gambhir. Combinatorial | + | 3. Ramasamy Paulmurugan, Sanjiv S. Gambhir. Combinatorial |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Latest revision as of 15:31, 27 September 2015
Coding sequence of Nluc398-FRB
N-luc398-FRB fusion protein ORF
Firefly (Photinus pyralis) luciferase can be split to N-terminal (N-luc) and C-terminal (C-luc) fragments and each of them is inactive. When they two reassembled non-covalently, the enzymatic activity would be reconstituted and the recovered luciferase is able to oxidize luciferin and produce detectable bioluminescence. Currently there are different combinations of split fragments, among which N-luc416/ C-luc398 and N-luc398/ C-luc394 are widely used[1].
Rapamycin-binding domain (FRB) of human mTOR (mammalian Target of Rapamycin) binds with high affinity to FK-506-binding protein 12 (FKBP). Previously Raik Gruenberg had already designed the part BBa_J18926, containing the coding sequence of FRB. Rapamycin is able to induce the dimerization to form a FRB-rapamycin-FKBP complex[2]. This protein-protein interaction can be visualized by split luciferase[3]. FRB and FKBP are fused to N-luc and C-luc respectively, and adding rapamycin can induce the approaching and reconstitution of split luciferase (Figure 1a).
2015 Peking iGEM improved the previous part BBa_J18926, they fused N-luc398 to N terminus of FRB (N-luc398-FRB, BBa_K1689004) and combined it with FKBP-Cluc394 (BBa_K1689006) to validate the functional reconstitution of split luciferase. However, compared with N-luc416/ C-luc398, the bioluminescence intensity didn't increase significantly after rapamycin was added (Figure 1). Therefore we discarded them and chose N-luc416/ C-luc398 as our split luciferase in the project (See BBa_K1689003 or BBa_K1689005).
Figure 1. Rapamycin-induced N-luc-FRB/ FKBP-C-luc complementation. (a) The working mechanism of rapamycin induced dimerization. The interacting protein partners (FRB & FKBP) get closer and dimerize soon after rapamycin is added (40nM) [3], thus to reconstitute the enzymatic activity of luciferase. (b) The experimental data. Error bars denote s.d.; n=3.
References
1. Ramasamy Paulmurugan, Sanjiv S. Gambhir. Firefly Luciferase Enzyme Fragment Complementation for Imaging in Cells and Living Animals. Anal Chem. 2005 March 1; 77(5): 1295–1302.
2. Rivera, V. M., T. Clackson, S. Natesan et al. A humanized system for pharmacologic control of gene expression. Nat. Med. 1996. 2:1028–1032.
3. Ramasamy Paulmurugan, Sanjiv S. Gambhir. Combinatorial