Difference between revisions of "Part:BBa K1720003"
| (22 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
This device with a GFP reporter was then transfected into HEK293 cells by lentiviral vector.Once we silence the PDE5A gene the level of cGMP will be up regulated as a result. The positive control was HEK293 cells that treat with Sodium Nitroprusside ,a NO donator that activate sGC and up regulate the level of cGMP. A negative control was made by transfecting an empty vector that does not contain scilencing device. We used Elisa to detect cGMP level. The results are as follow: | This device with a GFP reporter was then transfected into HEK293 cells by lentiviral vector.Once we silence the PDE5A gene the level of cGMP will be up regulated as a result. The positive control was HEK293 cells that treat with Sodium Nitroprusside ,a NO donator that activate sGC and up regulate the level of cGMP. A negative control was made by transfecting an empty vector that does not contain scilencing device. We used Elisa to detect cGMP level. The results are as follow: | ||
| − | |||
| Line 26: | Line 25: | ||
<partinfo>BBa_K1720003 parameters</partinfo> | <partinfo>BBa_K1720003 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | In the preliminary experiments, we designed a lentiviral vector containing our part and packaged the vector with lentivirus. The sequence to encode a fluorescent reporter was inserted after the antibiotics-resistance gene,rather than directly after our part, to avoid any potential influences on the function of the part. | ||
| + | |||
===Vector Map:=== | ===Vector Map:=== | ||
[[File:SCUT2015_China_shRNA1_vector.png|800px|thumb|left|]] | [[File:SCUT2015_China_shRNA1_vector.png|800px|thumb|left|]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| Line 126: | Line 121: | ||
===Virus Titer: (3.23±2)×10^8 TU/ml=== | ===Virus Titer: (3.23±2)×10^8 TU/ml=== | ||
| − | + | Functional titer was determined based on q-PCR amplification of a small fragment from the lentiviral vector, WRPE, which was integrated into the genome of transduced 293T cells. | |
===Experiment 1:=== | ===Experiment 1:=== | ||
| Line 151: | Line 146: | ||
<b>Result:</b> | <b>Result:</b> | ||
| − | [[File:SCUT China shRNA1.png|400px|thumb|left|]] | + | [[File:SCUT China shRNA1.png|400px|thumb|left|Green fluorescence signal after transfection of shRNA]] |
| − | |||
| − | |||
| − | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | The figure indicated that HEK293 cells had been transfected successfully! | ||
| + | |||
| + | |||
| + | ===Experiment 2:=== | ||
| + | |||
| + | After we proved that HEK293 cells can be transfected, expression levels of the PDE5A gene in the cells were determined by real-time PCR. | ||
| + | |||
<b>Protocol:</b> | <b>Protocol:</b> | ||
| + | |||
| + | <b>RNA extraction</b> | ||
| + | |||
| + | 1.Add trizol (3ml per culture dish); | ||
| + | |||
| + | 2.Keep portions in centrifuge tube(1ml per centrifuge tube) | ||
| + | |||
| + | 3.Homogenized by pipetting several times. | ||
| + | |||
| + | 4.Incubate samples for 5 min at room temp. | ||
| + | |||
| + | 5.Add chloroform (1/5 volume of trizol; e.g. 0.2ml to 1ml) | ||
| + | |||
| + | 6.Shake for 15sec. | ||
| + | |||
| + | 7.Incubate samples for 5 min at room temp. | ||
| + | |||
| + | 8.Centrifuge11.5G, 15 min, 4 ℃. | ||
| + | |||
| + | 9.Transfer 0.5ml aqueous phase to a new centrifuge tube. | ||
| + | |||
| + | 10.Add isopropanol (1/2 volume of trizol; e.g. 0.5ml to 1ml) | ||
| + | |||
| + | 11.Reverse blending. | ||
| + | |||
| + | 12.Incubate samples for 10 min at room temp. | ||
| + | |||
| + | 13.Centrifuge11.5G, 10 min, 4 ℃. | ||
| + | |||
| + | 14.Discard the supernatant. | ||
| + | |||
| + | 15.Add 70% EtOH (1 volume of trizol; e.g. 1ml to 1ml ,add & vortex briefly) | ||
| + | |||
| + | 16.Centrifuge11.5G, 5 min, 4 ℃. | ||
| + | |||
| + | 17.Discard the supernatant. | ||
| + | |||
| + | 18.Air-dry pellet for 2-5min. | ||
| + | |||
| + | 19.Add 20μlRNase free water and store in -70℃ environment. | ||
| + | |||
| + | 20.Determine RNA content by UV spectrophotometry. | ||
| + | |||
| + | 21.Electrophoresis of RNA. | ||
| + | |||
| + | |||
| + | <b>Two-Step RT-PCR</b> | ||
| + | |||
| + | STEP1:Reverse Transcription | ||
| + | |||
| + | 1.Assemble the reaction on ice. Add the enzyme last. | ||
| + | |||
| + | 2.Add the following components to a nuclease-free microcentrifuge tube. | ||
| + | [[File:SCUT2015_China_PCR1.jpg]] | ||
| + | |||
| + | 3.Heat mixture to 65°C for 5 min and quick chill on ice. Collect the contents of the tube by brief centrifugation and add: | ||
| + | [[File:SCUT2015_China_PCR2.jpg]] | ||
| + | |||
| + | 4.Mix contents of the tube gently and incubate at 37°C for 2 min. | ||
| + | |||
| + | 5.Add 1 µl (200 units) of M-MLV RT,and mix by pipetting gently up and down. | ||
| + | |||
| + | 6.Incubate 50 min at 37°C. | ||
| + | |||
| + | 7.Inactivate the reaction by heating at 70°C for 15 min. | ||
| + | |||
| + | STEP2:PCR | ||
| + | |||
| + | 1.Add RNase free water 3µl, PowerUp™ SYBR Green Master Mix 5µl, cDNA1µl, Forward primer 0.5µl, Reverse primer 0.5µl each tube in PCR Tubes Strip PCR. | ||
| + | |||
| + | 2) Mix the components gently but thoroughly. Then centrifuge brifly to spin down the cetents and eliminate any air bubbles.Incubate in Thermacycler: | ||
| + | |||
| + | |||
| + | a) UDG Activation: 50°C for 2min. | ||
| + | |||
| + | b)AmpliTaq Fast DNA Ploymerase,UP Activation: 95°C for 2min. | ||
| + | |||
| + | c) 40cycles: | ||
| + | |||
| + | |||
| + | |||
| + | Denature: 95°C for 15 sec | ||
| + | |||
| + | Anneal/Extend:60°C for 1min. | ||
<b>Result:</b> | <b>Result:</b> | ||
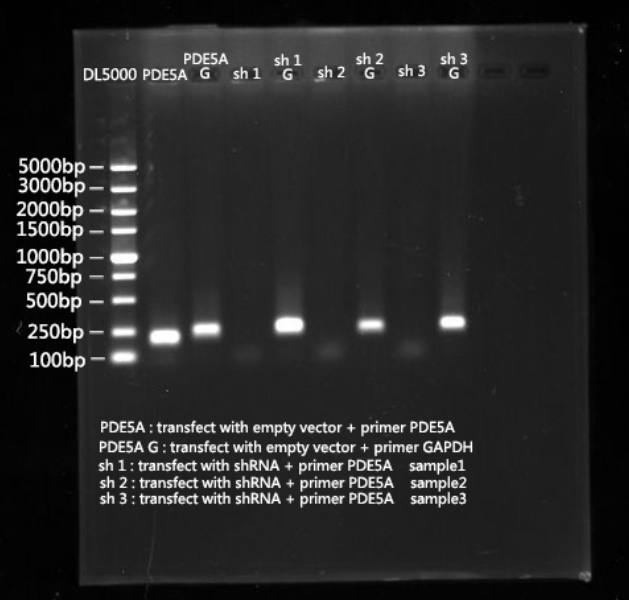
| + | [[File:SCUT_China_Gel_of_silencing__device.png|400px|thumb|left|PED5a transcription level after transfection with three different silencing devices]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
===Experiment 3:=== | ===Experiment 3:=== | ||
| − | After we | + | After we successfully suppressed PDE5A expression, we examined cellular cGMP level using an ELISA kit to examine whether our part can upregulate cGMP levels through PDE5A inhibition. |
<b>Protocol:</b> | <b>Protocol:</b> | ||
| − | 1. Prepare all standards and samples | + | 1. Prepare all standards and add samples in triplicate to the micro Elisa strip plate. |
2. Add standard : Set Standard wells , testing sample wells. Add standard 50 μl to standard well . | 2. Add standard : Set Standard wells , testing sample wells. Add standard 50 μl to standard well . | ||
| − | 3. Add testing sample 10 μl then add Sample Diluent 40 μl to testing sample well (samples were 5 times diluted ) ; Blank well doesn’t add | + | 3. Add Sample : Add testing sample 10 μl then add Sample Diluent 40 μl to testing sample well (samples were 5 times diluted ) ; Blank well doesn’t add anything. |
| − | 4. Add 100 μl of HRP-conjugate reagent to each well , cover with an adhesive strip and incubate for 60 minutes at | + | 4. Add 100 μl of HRP-conjugate reagent to each well , cover with an adhesive strip and incubate for 60 minutes at 37℃. |
5. Aspirate each well and wash by filling each well with Wash Solution (400μl ), repeating the process four times for a total of five washes. After the last wash, remove any remaining Wash Solution by decanting. Invert the plate and blot it against clean paper towels. | 5. Aspirate each well and wash by filling each well with Wash Solution (400μl ), repeating the process four times for a total of five washes. After the last wash, remove any remaining Wash Solution by decanting. Invert the plate and blot it against clean paper towels. | ||
| Line 184: | Line 326: | ||
7. Add 50μl Stop Solution to each well. | 7. Add 50μl Stop Solution to each well. | ||
| − | 8. Read the Optical Density ( | + | 8. Read the Optical Density (OD) at 450 nm using a Microplate Reader. |
| Line 195: | Line 337: | ||
<b>Result:</b> | <b>Result:</b> | ||
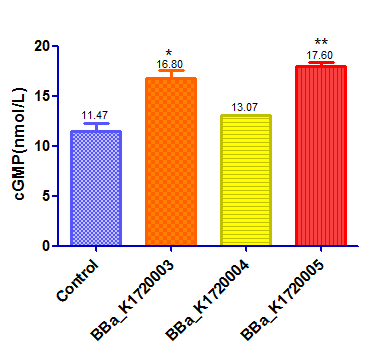
| − | [[File:SCUT_China_Elisa_of_scilencing__device.png|400px|thumb|left|]] | + | [[File:SCUT_China_Elisa_of_scilencing__device.png|400px|thumb|left|cGMP concentration after treated with three different silencing devices]] |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
===Experiment 4:=== | ===Experiment 4:=== | ||
| + | |||
| + | We used sodium nitroprusside(SNP) ,a NO donator that activate sGC and up regulate the level of cGMP, as a positive control. | ||
| + | |||
| + | <b>Protocol</b> | ||
| + | |||
| + | 1. Seed cells to be 90% confluent at 35mm culture dish. | ||
| + | |||
| + | 2. Dilute 20ul SNP (0.1ul mol/L) and 20ul cysteine (0.2ul mol/L) in 1960ul DMEM medium containing 10% FBS | ||
| + | |||
| + | 3.Withdraw culture medium from 35mm culture dish | ||
| + | |||
| + | 4.Add the mixture in step 2 to the cells | ||
| + | |||
| + | 5. Incubate for 10min,20min,30min,60min,90min | ||
| + | |||
| + | 6. Use cGMP Elisa kit to detect the cGMP concentration | ||
| + | |||
| + | |||
| + | <b>Note:</b> | ||
| + | |||
| + | 1. We used cysteine to help SNP release NO | ||
| + | |||
| + | 2.Cysteine will cause interference when we use BCA protein assay kit to detect total protein level as a internal reference, so we make two control. One of the control was treat by the same amount of cysteine,the other was treat with DMEM medium to cut down the background caused by cysteine. | ||
| + | |||
| + | |||
| + | <b>Result:</b> | ||
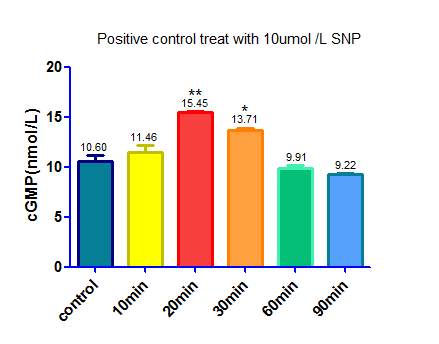
| + | [[File: SCUT_China_Elisa_of_SNP.png|400px|thumb|left| cGMP concentration after treated with 10umol/L SNP]] | ||
Latest revision as of 02:51, 18 September 2015
Human phosphodiesterase 5A gene silencing device NO.1
This device is uesd for silencing the human phosphodiesterase 5A (PDE5A) gene.A U6 promoter driving a designed, synthetic shRNA-like miRNA followed by the terminator.
PDE5A is a cGMP-binding, cGMP-specific phosphodiesterase, a member of the cyclic nucleotide phosphodiesterase family. This phosphodiesterase specifically hydrolyzes cGMP to 5'-GMP. It is involved in the regulation of intracellular concentrations of cyclic nucleotides and is important for smooth muscle relaxation in the cardiovascular system.
We designed 3 silencing device and test their function at the same time.Here is the another two device :BBa_K1720004,BBaK172005
This device with a GFP reporter was then transfected into HEK293 cells by lentiviral vector.Once we silence the PDE5A gene the level of cGMP will be up regulated as a result. The positive control was HEK293 cells that treat with Sodium Nitroprusside ,a NO donator that activate sGC and up regulate the level of cGMP. A negative control was made by transfecting an empty vector that does not contain scilencing device. We used Elisa to detect cGMP level. The results are as follow:
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 273
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 247
- 1000COMPATIBLE WITH RFC[1000]
In the preliminary experiments, we designed a lentiviral vector containing our part and packaged the vector with lentivirus. The sequence to encode a fluorescent reporter was inserted after the antibiotics-resistance gene,rather than directly after our part, to avoid any potential influences on the function of the part.
Vector Map:
Vector Components:
Virus Titer: (3.23±2)×10^8 TU/ml
Functional titer was determined based on q-PCR amplification of a small fragment from the lentiviral vector, WRPE, which was integrated into the genome of transduced 293T cells.
Experiment 1:
At the beginning of our experiment, we aimed to prove that HEK293 cells can be transfected by our vector. In our vector we inserted EGFP gene as a repoter.Once HEK293 cells are transfected successfully green fluorescence signal will be observed under fluorescence microscope.
Protocol: 1. Seed cells to be 40% confluent at a 35mm culture dish.
2. Dilute 10ul lentiviral vector in 1ml DMEM medium containing 10% FBS
3. Withdraw culture medium from 35mm culture dish.
4. Add vector-DMEM complex to cells
5. Incubate for 15 hours.
6. Withdraw vector-DMEM complex from culture dish.
7. Add 2ml DMEM medium containing 10% FBS to cells and incubate for 10 hours
8. Observe the cells under Inverted fluorescence microscope.
Result:
The figure indicated that HEK293 cells had been transfected successfully!
Experiment 2:
After we proved that HEK293 cells can be transfected, expression levels of the PDE5A gene in the cells were determined by real-time PCR.
Protocol:
RNA extraction
1.Add trizol (3ml per culture dish);
2.Keep portions in centrifuge tube(1ml per centrifuge tube)
3.Homogenized by pipetting several times.
4.Incubate samples for 5 min at room temp.
5.Add chloroform (1/5 volume of trizol; e.g. 0.2ml to 1ml)
6.Shake for 15sec.
7.Incubate samples for 5 min at room temp.
8.Centrifuge11.5G, 15 min, 4 ℃.
9.Transfer 0.5ml aqueous phase to a new centrifuge tube.
10.Add isopropanol (1/2 volume of trizol; e.g. 0.5ml to 1ml)
11.Reverse blending.
12.Incubate samples for 10 min at room temp.
13.Centrifuge11.5G, 10 min, 4 ℃.
14.Discard the supernatant.
15.Add 70% EtOH (1 volume of trizol; e.g. 1ml to 1ml ,add & vortex briefly)
16.Centrifuge11.5G, 5 min, 4 ℃.
17.Discard the supernatant.
18.Air-dry pellet for 2-5min.
19.Add 20μlRNase free water and store in -70℃ environment.
20.Determine RNA content by UV spectrophotometry.
21.Electrophoresis of RNA.
Two-Step RT-PCR
STEP1:Reverse Transcription
1.Assemble the reaction on ice. Add the enzyme last.
2.Add the following components to a nuclease-free microcentrifuge tube.

3.Heat mixture to 65°C for 5 min and quick chill on ice. Collect the contents of the tube by brief centrifugation and add:
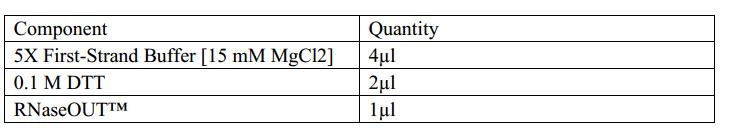
4.Mix contents of the tube gently and incubate at 37°C for 2 min.
5.Add 1 µl (200 units) of M-MLV RT,and mix by pipetting gently up and down.
6.Incubate 50 min at 37°C.
7.Inactivate the reaction by heating at 70°C for 15 min.
STEP2:PCR
1.Add RNase free water 3µl, PowerUp™ SYBR Green Master Mix 5µl, cDNA1µl, Forward primer 0.5µl, Reverse primer 0.5µl each tube in PCR Tubes Strip PCR.
2) Mix the components gently but thoroughly. Then centrifuge brifly to spin down the cetents and eliminate any air bubbles.Incubate in Thermacycler:
a) UDG Activation: 50°C for 2min.
b)AmpliTaq Fast DNA Ploymerase,UP Activation: 95°C for 2min.
c) 40cycles:
Denature: 95°C for 15 sec
Anneal/Extend:60°C for 1min.
Result:
Experiment 3:
After we successfully suppressed PDE5A expression, we examined cellular cGMP level using an ELISA kit to examine whether our part can upregulate cGMP levels through PDE5A inhibition.
Protocol:
1. Prepare all standards and add samples in triplicate to the micro Elisa strip plate.
2. Add standard : Set Standard wells , testing sample wells. Add standard 50 μl to standard well .
3. Add Sample : Add testing sample 10 μl then add Sample Diluent 40 μl to testing sample well (samples were 5 times diluted ) ; Blank well doesn’t add anything.
4. Add 100 μl of HRP-conjugate reagent to each well , cover with an adhesive strip and incubate for 60 minutes at 37℃.
5. Aspirate each well and wash by filling each well with Wash Solution (400μl ), repeating the process four times for a total of five washes. After the last wash, remove any remaining Wash Solution by decanting. Invert the plate and blot it against clean paper towels.
6. Add chromogen solution A 50μl and chromogen solution B 50μl to each well.Gently mix and incubate for 15 minutes at 37 C . Protect from light .
7. Add 50μl Stop Solution to each well.
8. Read the Optical Density (OD) at 450 nm using a Microplate Reader.
Note:
1.Standard ( S0 → S5 ) concentration was followed by: 0,2,4,8,16,32 nmol/L.
2.We used BCA protein assay kit to detect total protein level as a internal reference and the result was corrected by it.
Result:
Experiment 4:
We used sodium nitroprusside(SNP) ,a NO donator that activate sGC and up regulate the level of cGMP, as a positive control.
Protocol
1. Seed cells to be 90% confluent at 35mm culture dish.
2. Dilute 20ul SNP (0.1ul mol/L) and 20ul cysteine (0.2ul mol/L) in 1960ul DMEM medium containing 10% FBS
3.Withdraw culture medium from 35mm culture dish
4.Add the mixture in step 2 to the cells
5. Incubate for 10min,20min,30min,60min,90min
6. Use cGMP Elisa kit to detect the cGMP concentration
Note:
1. We used cysteine to help SNP release NO
2.Cysteine will cause interference when we use BCA protein assay kit to detect total protein level as a internal reference, so we make two control. One of the control was treat by the same amount of cysteine,the other was treat with DMEM medium to cut down the background caused by cysteine.
Result: