Difference between revisions of "Part:BBa K1666000"
(→Usage and Biology) |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1666000 short</partinfo> | <partinfo>BBa_K1666000 short</partinfo> | ||
| − | Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of | + | Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of signaling molecules named autoinducers. And autoinducer-2 (AI-2) serves as a 'universal signal' for interspecies communication. In the LuxS/AI-2 signaling system of ''Salmonella Typhimurium'', AI-2 response involves ATP binding cassette transporter encoded by genes named ''lsr'' (LuxS regulated). And LsrA is ATP-binding protein that provides energy for AI-2 transport. |
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | + | AI-2 is generated by many species of Gram-negative and Gram-positive bacteria. In a group of bacteria exemplified by ''Salmonella'', AI-2 response involves ''lsr'' genes that encode ATP binding cassette-type transporter. And LsrA is part of the ABC transporter complex, being responsible for energy coupling to the transport system. | |
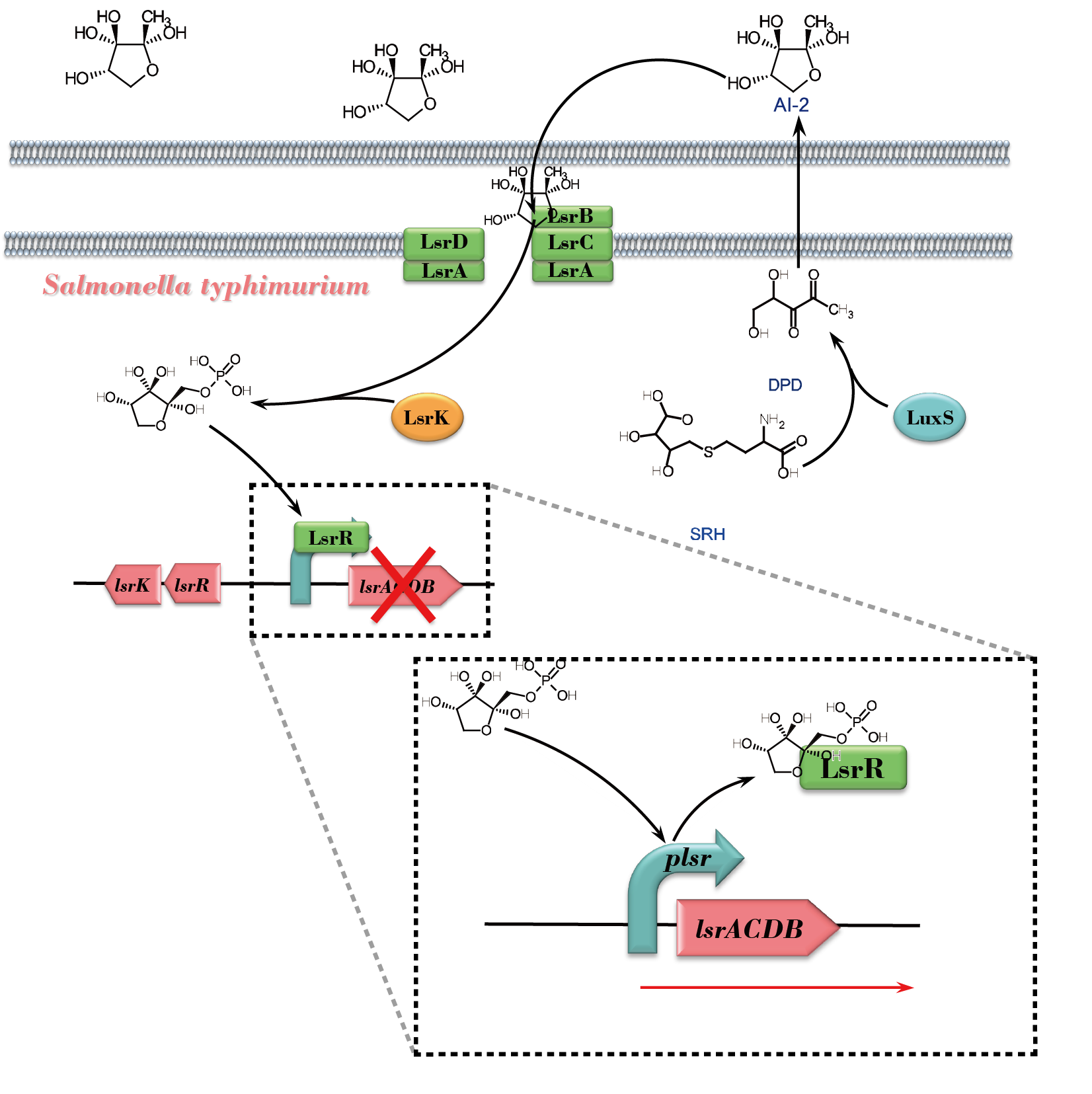
| − | + | [[File:NEFU_China_2015_AI-2_response_in_Salmonalla.png|550px|thumb|center|'''Fig1. Schematic overview of the AI-2 response pathway in ''Salmonella Typhimurium'''''The precursor of AI-2, 4,5-Dihydroxy-2,3-Pentanedione (DPD) , is a byproduct generated when LuxS converts S-Ribosylhomocysteine (SRH) to Homocysteine (HCY). DPD then undergoes spontaneously cyclization, forming AI-2, and exports to the culture supernatant. After that, extracellular AI-2 bounds to LsrB, following by passing the membrane channel and importing the cytoplasm. LsrK phosphorylates AI-2 afterwards. The ''lsr'' operon is repressed until phosphorylated AI-2 causes LsrR to relieve its repression on the promoter. And this allows further AI-2 import.]] | |
| − | + | In our project, we set this protein-coding part under the regulation of a nisA promoter which can be activated by food-grade inducer, nisin. We linearized the related expression vectors and stably integrated them into the genome of the hosts. And together with other parts, we will construct a membrane channel for AI-2 generated by pathogens in the engineered bacteria. | |
| − | + | [[File:NEFU_China_2015_pnisA_and_LsrA.png|450px|thumb|center|'''Fig2. Part of the vector containing ''lsrA'' '''We use nisA promoter to initiate the transcription of ''lsrA'' gene]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | [[File: | + | |
<!-- --> | <!-- --> | ||
| − | <span class='h3bb'>Sequence and Features</span> | + | <span class='h3bb'>Sequence and Features</span>expre |
<partinfo>BBa_K1666000 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1666000 SequenceAndFeatures</partinfo> | ||
Latest revision as of 14:49, 6 September 2015
LsrA of LuxS/AI-2 signaling pathway in Salmonalla
Quorum sensing is a process of bacterial cell-to-cell communication involving the production and detection of signaling molecules named autoinducers. And autoinducer-2 (AI-2) serves as a 'universal signal' for interspecies communication. In the LuxS/AI-2 signaling system of Salmonella Typhimurium, AI-2 response involves ATP binding cassette transporter encoded by genes named lsr (LuxS regulated). And LsrA is ATP-binding protein that provides energy for AI-2 transport.
Usage and Biology
AI-2 is generated by many species of Gram-negative and Gram-positive bacteria. In a group of bacteria exemplified by Salmonella, AI-2 response involves lsr genes that encode ATP binding cassette-type transporter. And LsrA is part of the ABC transporter complex, being responsible for energy coupling to the transport system.
In our project, we set this protein-coding part under the regulation of a nisA promoter which can be activated by food-grade inducer, nisin. We linearized the related expression vectors and stably integrated them into the genome of the hosts. And together with other parts, we will construct a membrane channel for AI-2 generated by pathogens in the engineered bacteria.
Sequence and Featuresexpre
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 939
- 1000COMPATIBLE WITH RFC[1000]

