Difference between revisions of "Part:BBa J45992:Experience"
(→Applications of BBa_J45992) |
Jdesca911e (Talk | contribs) (→User Reviews) |
||
| (27 intermediate revisions by 5 users not shown) | |||
| Line 4: | Line 4: | ||
===Applications of BBa_J45992=== | ===Applications of BBa_J45992=== | ||
| − | + | A stationary phase-dependent PoPS source. | |
| − | + | ||
| − | + | ||
| − | + | ||
===User Reviews=== | ===User Reviews=== | ||
| Line 21: | Line 18: | ||
|}; | |}; | ||
<!-- End of the user review template --> | <!-- End of the user review template --> | ||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_J45992 AddReview 5</partinfo><br> | ||
| + | <I>[[User:Rshetty|Reshma Shetty]]</I> | ||
| + | |width='60%' valign='top'| | ||
| + | <partinfo>BBa_J45992</partinfo>, when used to regulate transcription of GFP in <partinfo>BBa_J45995</partinfo> or banana odor enzyme generator in <partinfo>BBa_J45250</partinfo>, demonstrated the expected behavior. <partinfo>BBa_J45992</partinfo> produced a low transcriptional signal in exponential phase and a high transcriptional signal in stationary phase. | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_J45992 AddReview 5</partinfo> | ||
| + | <I>Jean Descarpentrie</I> | ||
| + | |width='60%' valign='top'| | ||
| + | <partinfo>BBa_J45992</partinfo> was no longer in stock so we submitted it once again. We used it to express our Curdlan synthase gene (crdS) <partinfo>BBa_K1686046 </partinfo> in stationary phase. <partinfo>BBa_J45992</partinfo> worked as expected, activating our gene only in stationary phase. | ||
| + | |}; | ||
| + | <!-- End of the user review template --> | ||
| + | |||
| + | |||
| + | |||
| + | |||
<!-- DON'T DELETE --><partinfo>BBa_J45992 EndReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_J45992 EndReviews</partinfo> | ||
| + | |||
| + | ==Characterization== | ||
| + | |||
| + | ===Transcriptional control of GFP generator=== | ||
| + | {{:iGEM:MIT/2006/Transcriptional control devices}} | ||
| + | |||
| + | <br style="clear:both;"/> | ||
| + | |||
| + | ===Transcriptional control of banana odor enzyme generator=== | ||
| + | |||
| + | {{:IGEM:MIT/2006/Growth dependent banana odor generator}} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-- ===Transcriptional control of Curdlan synthase (crdS)=== --> | ||
| + | <br style="clear:both;"/> | ||
| + | {{:iGEM:Bordeaux/2015/stationary phase curdlan synthase}} | ||
Latest revision as of 07:33, 18 September 2015
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J45992
A stationary phase-dependent PoPS source.
User Reviews
UNIQ614889fc77890d84-partinfo-00000000-QINU
|
••••• |
BBa_J45992, when used to regulate transcription of GFP in BBa_J45995 or banana odor enzyme generator in BBa_J45250, demonstrated the expected behavior. BBa_J45992 produced a low transcriptional signal in exponential phase and a high transcriptional signal in stationary phase. |
|
•••••
Jean Descarpentrie |
BBa_J45992 was no longer in stock so we submitted it once again. We used it to express our Curdlan synthase gene (crdS) BBa_K1686046 in stationary phase. BBa_J45992 worked as expected, activating our gene only in stationary phase. |
UNIQ614889fc77890d84-partinfo-0000000A-QINU
Characterization
Transcriptional control of GFP generator

We successfully designed, constructed and tested transcriptional control devices for constitutive, stationary phase dependent and exponential phase dependent protein production (A-C). To test and verify function of our three transcriptional control devices, we assembled each control device with the GFP protein generator BBa_E0840 and monitored the fluorescence of E. coli cultures with each device over time. For each device, we plot the change in fluorescence per unit time (normalized GFP synthesis rate) versus the cell density (OD600nm) (D). The constitutive transcriptional control device produced a high GFP synthesis rate irrespective of cell density. The stationary phase transcriptional control device produced a low initial GFP synthesis rate which increased with culture cell density. The exponential phase transcriptional control device produced an initially high GFP synthesis rate which dropped off as cell density increased. Data shown are averages of triplicate measurements of cultures grown from three individual colonies of each device. Error bars are the standard deviation of the three individual cultures.
Transcriptional control of banana odor enzyme generator
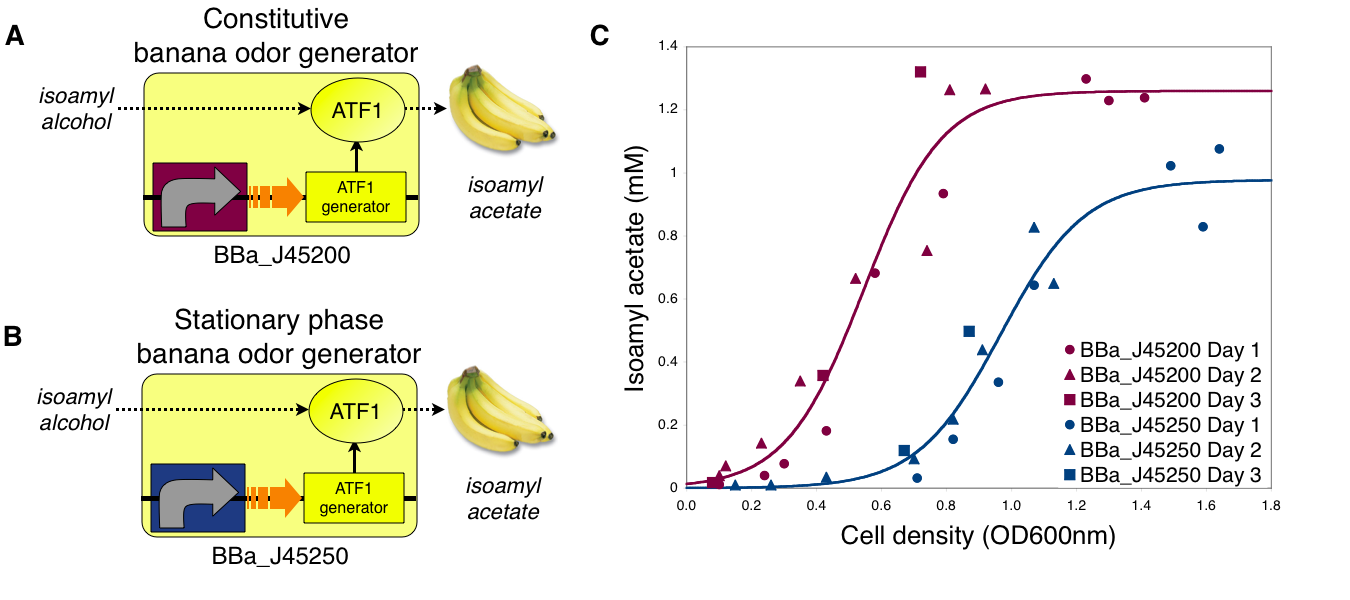
To demonstrate growth phase dependent banana odor production, we compared the behavior of constitutive and stationary phase dependent banana odor generators (A and B, respectively). We measured isoamyl acetate concentrations of cultures of the constitutive and stationary phase banana odor generators at different culture cell densities (OD600nm) (C). As expected, the stationary phase banana odor generator produced very little isoamyl acetate at low cell densities but its isoamyl acetate production increased with cell density. By comparison, the constitutive banana odor generator produced more isoamyl acetate at lower cell densities than the stationary phase banana odor generator. To visually aid comparison of the two odor generators, an empirical fit to the data for each device is shown.

Curdlan synthase under the expression of stationary phase promoter
To demonstrate growth phase dependent activation of our Curdlan synthase gene, we compared the amount of Curdlan produced by E.coli while at the same time looking at OD to measure growth. The Curdlan concentration was calculated with absorption measures (excitation wavelength at 398nm and emission wavelength= 502nm) after Curdlan purification from cells. We used aniline blue dye since it is specific of beta glucans.
