Difference between revisions of "Part:BBa K1321346"
| (One intermediate revision by the same user not shown) | |||
| Line 8: | Line 8: | ||
Note that the start and stop codon, plus 6 bp either side of the sequence, are included the RFC25 prefix and suffix which is not shown. | Note that the start and stop codon, plus 6 bp either side of the sequence, are included the RFC25 prefix and suffix which is not shown. | ||
| + | As part of our project we carried out an assay to determine the relative binding ability of CBD-sfGFP and sfGFP fusions to bacterial cellulose. | ||
| + | |||
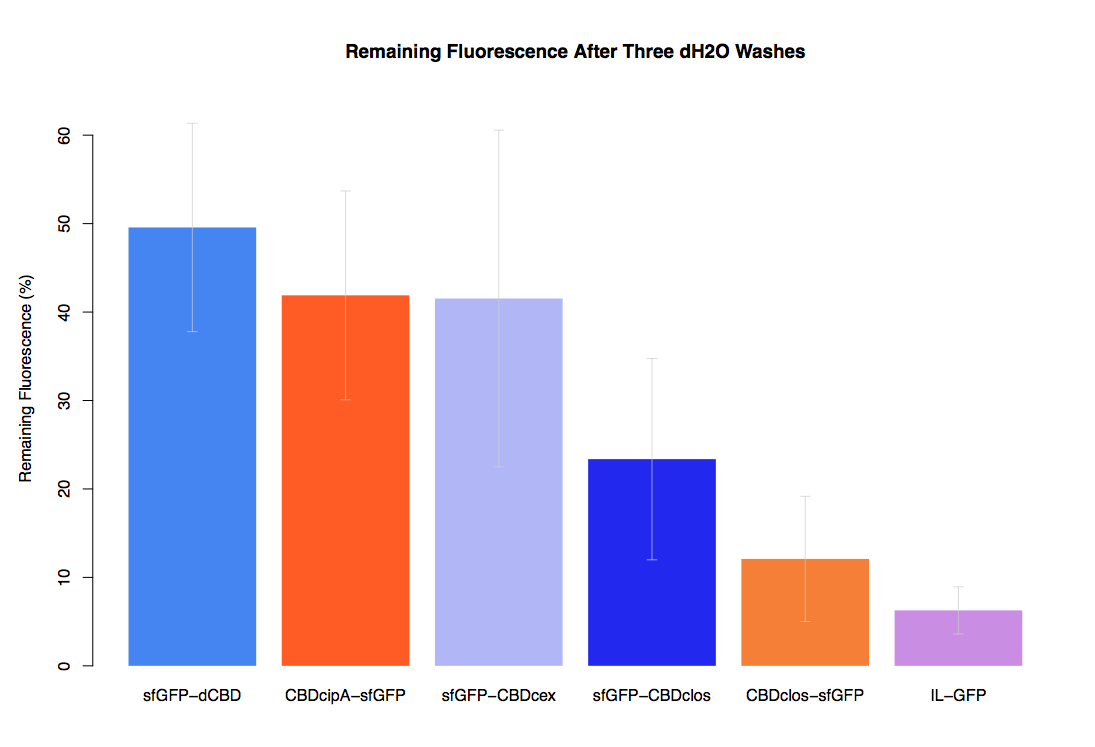
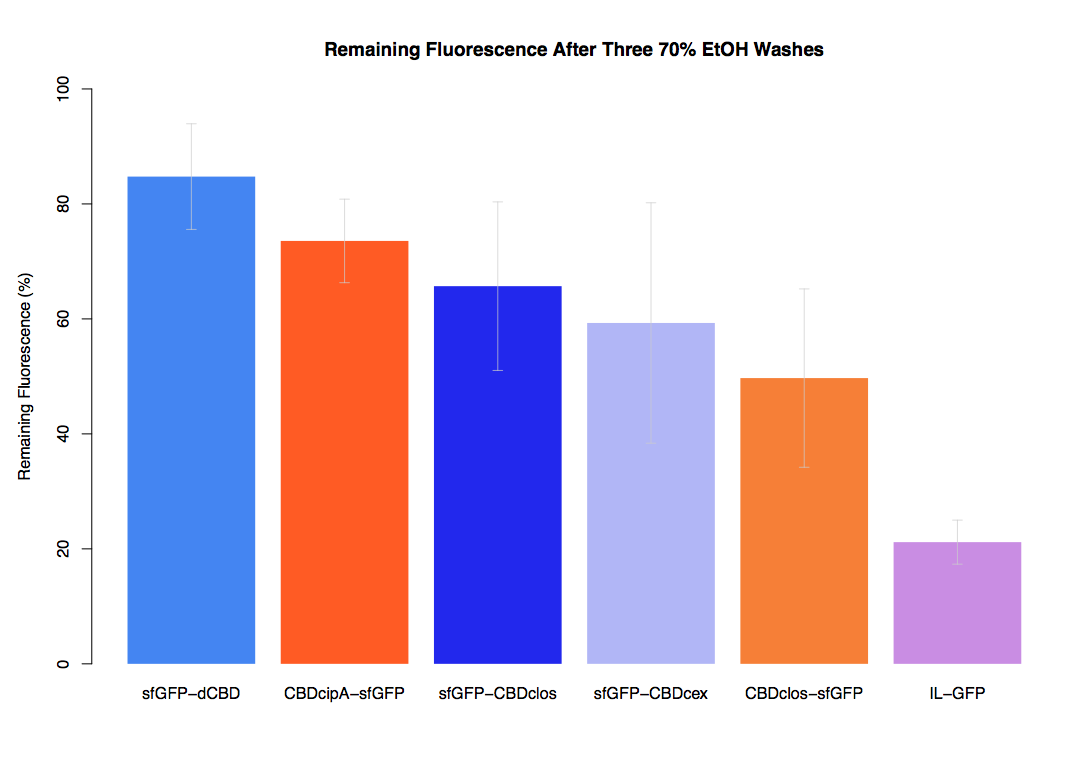
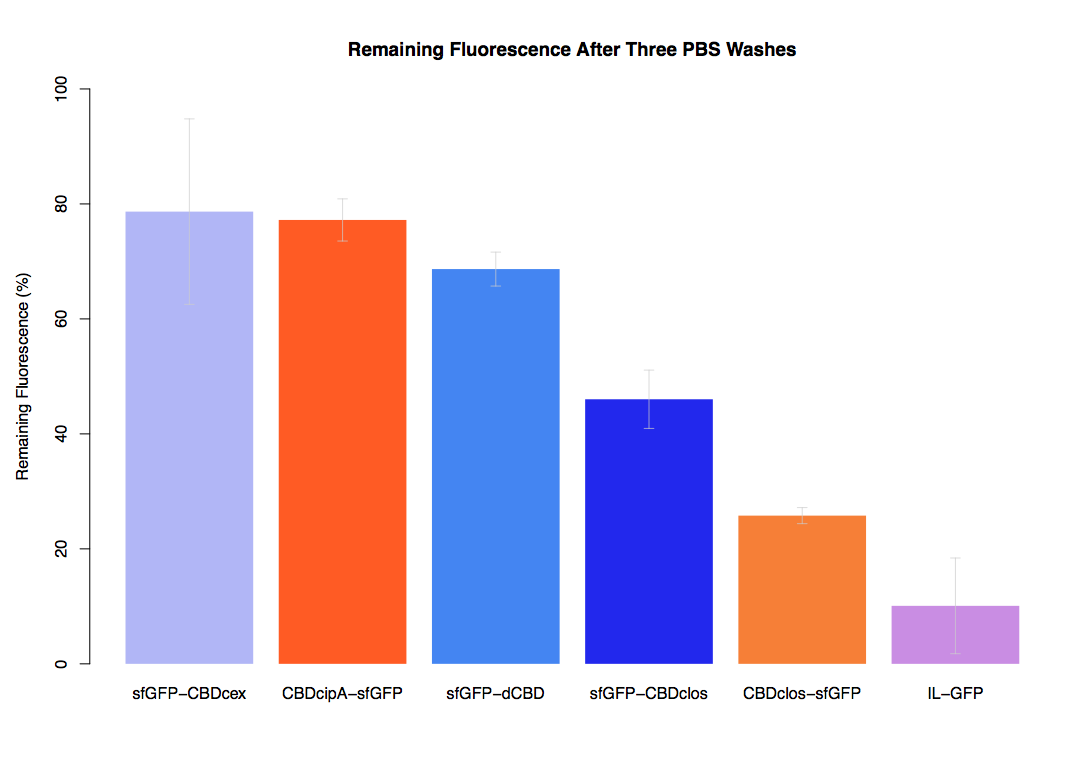
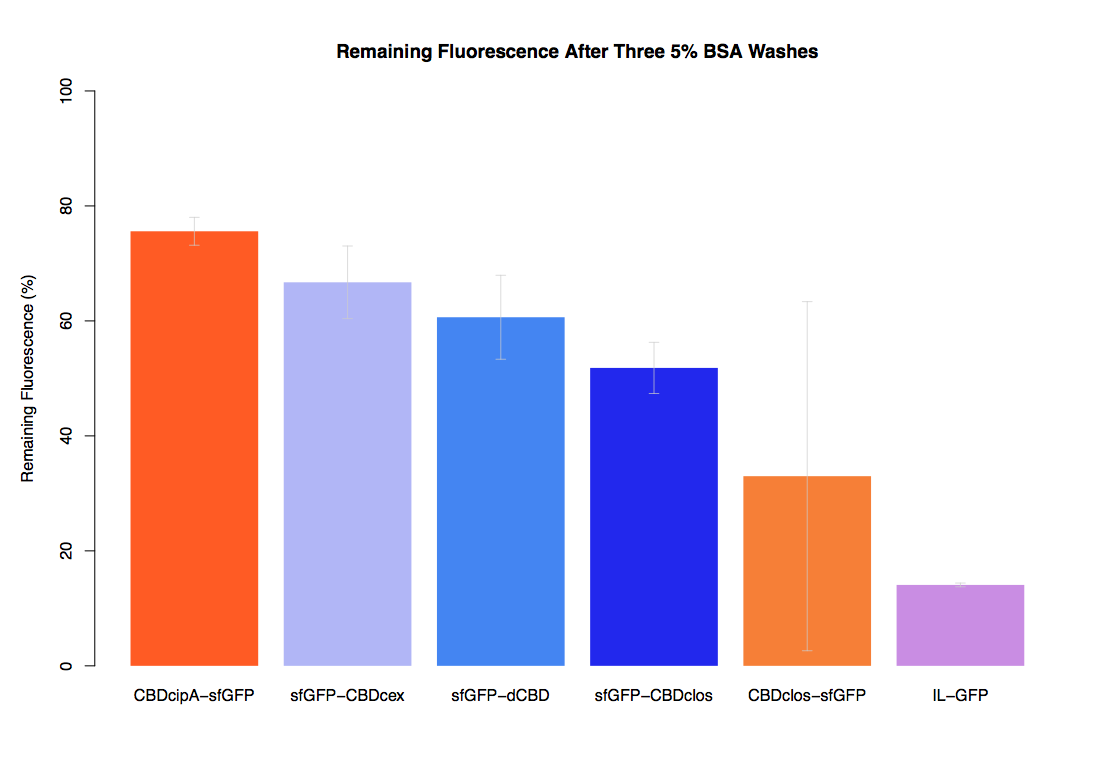
| + | Our first assay was performed to determine the relative strengths of various CBDs’ binding to bacterial cellulose. These were represented by the percentage fluorescence left from CBDs fused to ([https://parts.igem.org/Part:BBa_K1321337 sfGFP (RFC25)]) bound to bacterial cellulose discs, when subjected to various washes. Full protocol can be found ([http://2014.igem.org/Team:Imperial/Protocols here]) | ||
| + | |||
| + | In the same assay, results suggested that dCBD, CBDcex, CBDcipA, and C-terminal CBDclos (all fused to sfGFP) had greater ability to bind bacterial cellulose than N-terminal CBDclos. Three washes with dH2O, PBS and 5% BSA were carried out to monitor binding strength. It was determined that the fusion with N-terminal CBDclos was, on average, our fifth strongest CBD, in comparison other CBDs tested. | ||
| + | |||
| + | |||
| + | [[File:IC14_-_dH2Obplot1.png|600px|thumb|left|Figure 1 - CBD binding strength after three dH2O washes. ]] | ||
| + | |||
| + | [[File:IC14-EtOHbplot1.png|600px|thumb|left|Figure 2 - CBD binding strength after three 70%Ethanol washes]] | ||
| + | |||
| + | [[File:IC14-PBSbplot1.png|600px|thumb|left|Figure 3 - CBD binding strength after three PBS washes]] | ||
| + | |||
| + | [[File:IC14-BSAbplot1.png|600px|thumb|left|Figure 4 - CBD binding strength after three BSA washes]] | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 13: | Line 27: | ||
<!-- --> | <!-- --> | ||
| − | |||
<partinfo>BBa_K1321346 SequenceAndFeatures</partinfo> | <partinfo>BBa_K1321346 SequenceAndFeatures</partinfo> | ||
Latest revision as of 05:50, 2 November 2014
CBDclos fused to sfGFP in RFC 25
super-folder GFP fused C-terminally to CBDclos (a cellulose-binding domain), which contains an endogenous N-terminal linker sequence.
This construct is part of a library of Super-folder GFP fusions with cellulose binding domains, which we used to assay the CBD binding affinity. Please see our [http://2014.igem.org/Team:Imperial/Functionalisation project page] for more information. The collection of sfGFP-CBD fusion parts can be seen in the table below: 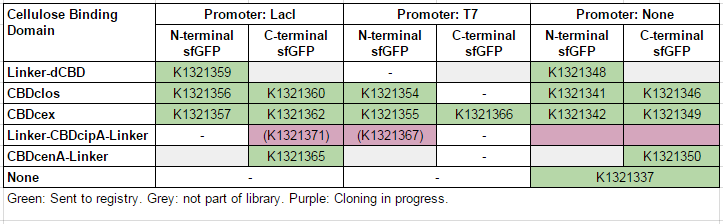
Note that the start and stop codon, plus 6 bp either side of the sequence, are included the RFC25 prefix and suffix which is not shown.
As part of our project we carried out an assay to determine the relative binding ability of CBD-sfGFP and sfGFP fusions to bacterial cellulose.
Our first assay was performed to determine the relative strengths of various CBDs’ binding to bacterial cellulose. These were represented by the percentage fluorescence left from CBDs fused to (sfGFP (RFC25)) bound to bacterial cellulose discs, when subjected to various washes. Full protocol can be found ([http://2014.igem.org/Team:Imperial/Protocols here])
In the same assay, results suggested that dCBD, CBDcex, CBDcipA, and C-terminal CBDclos (all fused to sfGFP) had greater ability to bind bacterial cellulose than N-terminal CBDclos. Three washes with dH2O, PBS and 5% BSA were carried out to monitor binding strength. It was determined that the fusion with N-terminal CBDclos was, on average, our fifth strongest CBD, in comparison other CBDs tested.
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 316