Difference between revisions of "Part:BBa K1509001"
| (19 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K1509001 short</partinfo> | <partinfo>BBa_K1509001 short</partinfo> | ||
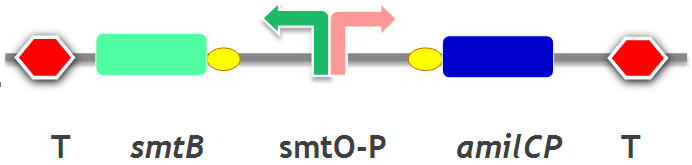
| − | smt O-P is a promoter which has two inverted repeats, designated S1/S2 and S3/S4, in the overlapping promoter/operator sites between gene smtA and gene smtB. The full promoter/operator DNA binds two SmtB dimmers forming a S1/S2-SmtB:SmtB-S3/S4 bridge complex. After binding with smtB, | + | smt O-P is a promoter which has two inverted repeats, designated S1/S2 and S3/S4, in the overlapping promoter/operator sites between gene smtA and gene smtB. The full promoter/operator DNA binds two SmtB dimmers forming a S1/S2-SmtB:SmtB-S3/S4 bridge complex. After binding with smtB, the expression of downstream gene (smtA) is repressed. |
| + | |||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | In our project, we wanted to use pigments as reporters in our designed genetic construction which can be recognizable by the naked eyes. According to the previous work, we have chosen an identified pigment in the Registry: the biobrick of amilCP (BBa_K592009) was used as reporter gene in our metal detection device. After exchanging the biobrick part with SmtA in smtB-OP-smtA device, the pigment gene was under control of metal-induced promoter (smtB-OP). | ||
| + | [[File:Fig3.smtB-OP-amilCP.png]] | ||
| + | |||
| + | |||
| + | ===Result=== | ||
| + | In summary, the ''Rosetta-plysS'' strain made our system convenient to be applied, the classical smtB-OP-smtA device from ''Staphylococcus'' supported our system a responsive Cd<sup>2+</sup> inducible-promoter, and the viewable pigment gene provided our system a reliable and macroscopic observation. After theoretical prediction, genetic engineering, experimental optimization and reasonable model analysis (deeply discussed in Modeling), our detecting system residing in the engineering bacteria was able to sensitively represent the content of Cd<sup>2+</sup> (1-100μM) in 1-2 hours. | ||
| + | |||
| + | Although the inducible operator in our case might also response to other metal ions including Zn<sup>2+</sup>, our date at least did point out that Cd<sup>2+</sup> has acuter stimulus to the pigment gene than Zn<sup>2+</sup> which was confirmed both from experimental data and model analysis. We achieved to our goal at a certain degree. Finally, our system is easier to utilize and exhibits improved flexibility as a tool to detect Cd<sup>2+</sup> which belongs to the toxical heavy metal ions. | ||
| + | |||
| + | [[File:Re-NEFU_CHINA_detecting_fig1.png]] | ||
| + | |||
| + | |||
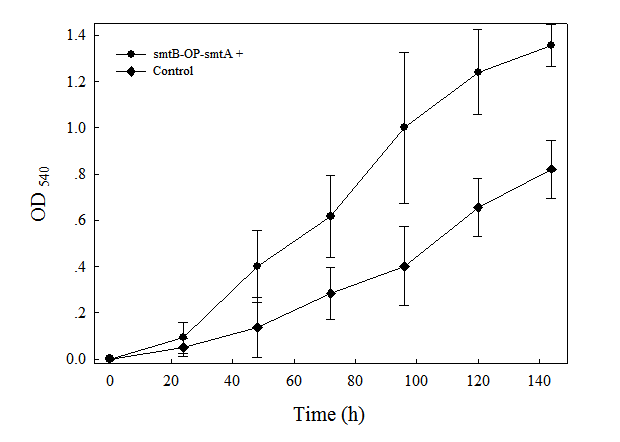
| + | fig.1. Growth of cells containing smtB-OP-smtA element in LB medium supplemented with 2μM Cd<sup>2+</sup>. Cells were inoculated at a density of 1x10<sup>6</sup> cells ml<sup>-1</sup>, and growth was monitored by measuring the OD540 value. Data points represent the mean values from three separate cultures with SD. | ||
| + | |||
| + | [[File:Detecting_fig2.png]] | ||
| + | |||
| + | |||
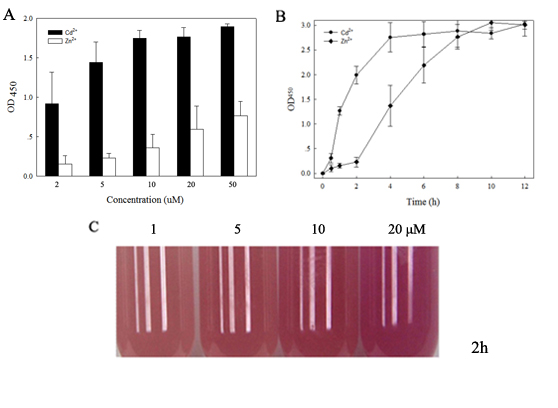
| + | fig.2. A. Metal-induced expression of the pigments (RFP), ''Rosetta-plysS'' (1x10<sup>6</sup> cells ml<sup>-1</sup>) carrying the smtB-OP-RFP element were grown with Cd<sup>2+</sup> and Zn<sup>2+</sup> (1-50μM) supplement for 2h immediately before assay and expression was monitored by measuring the OD450 value; B. Metal-induced expression of the pigments (RFP). Rosetta-plysS carrying the smtB-OP-RFP element were grown with Cd<sup>2+</sup> and Zn<sup>2+</sup> (2μM) supplement for 1-12h immediately before measurement; C. Cadmium-induced expression of the pigment (RFP) at different concentrations. Rosetta-plysS (1x10<sup>7</sup> cells ml<sup>-1</sup>) carrying the smtB-OP-RFP element were grown with Cd<sup>2+</sup> (1-20μM) supplement for 2h. The data points shown in A and B represent the means of three separate assays with SD. | ||
| + | |||
| + | [[File:Detecting_fig3.png]] | ||
| + | |||
| + | |||
| + | |||
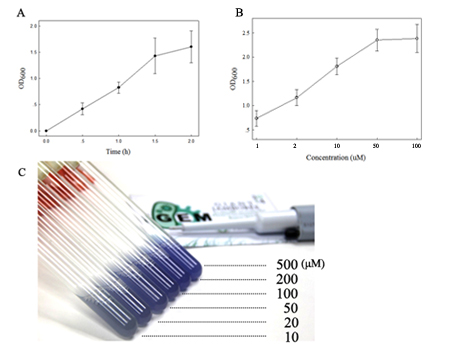
| + | fig.3. A. Cadmium-induced expression of the pigment (amilCP) at constant concentration. ''Rosetta-plysS'' (1x10<sup>6</sup> cells ml<sup>-1</sup>) carrying the smtB-OP-amilCP element were grown with Cd<sup>2+</sup> (1μM) supplement for 1-2h immediately before assay and the expression was monitored by measuring the OD600 value; B. Cadmium-induced expression of the pigment (amilCP) with different concentrations. ''Rosetta-plysS'' carrying the smtB-OP-amilCP device were grown with Cd<sup>2+</sup> (1-100μM) supplement for 1h immediately before assay;C. Cadmium-induced expression of the pigment (amilCP) with different concentrations. ''Rosetta-plysS'' (1x10<sup>7</sup> cells ml<sup>-1</sup>) carrying the smtB-OP-amilCP element were grown with Cd<sup>2+</sup> (10, 20, 50, 100, 200 and 500 μM) supplement for 2h. The data points shown in A and B represent the means of three separate values with SD. | ||
| + | |||
| + | |||
<!-- --> | <!-- --> | ||
Latest revision as of 13:34, 17 October 2014
A bi-directional promoter affected by SmtB protein
smt O-P is a promoter which has two inverted repeats, designated S1/S2 and S3/S4, in the overlapping promoter/operator sites between gene smtA and gene smtB. The full promoter/operator DNA binds two SmtB dimmers forming a S1/S2-SmtB:SmtB-S3/S4 bridge complex. After binding with smtB, the expression of downstream gene (smtA) is repressed.
Usage and Biology
In our project, we wanted to use pigments as reporters in our designed genetic construction which can be recognizable by the naked eyes. According to the previous work, we have chosen an identified pigment in the Registry: the biobrick of amilCP (BBa_K592009) was used as reporter gene in our metal detection device. After exchanging the biobrick part with SmtA in smtB-OP-smtA device, the pigment gene was under control of metal-induced promoter (smtB-OP).
Result
In summary, the Rosetta-plysS strain made our system convenient to be applied, the classical smtB-OP-smtA device from Staphylococcus supported our system a responsive Cd2+ inducible-promoter, and the viewable pigment gene provided our system a reliable and macroscopic observation. After theoretical prediction, genetic engineering, experimental optimization and reasonable model analysis (deeply discussed in Modeling), our detecting system residing in the engineering bacteria was able to sensitively represent the content of Cd2+ (1-100μM) in 1-2 hours.
Although the inducible operator in our case might also response to other metal ions including Zn2+, our date at least did point out that Cd2+ has acuter stimulus to the pigment gene than Zn2+ which was confirmed both from experimental data and model analysis. We achieved to our goal at a certain degree. Finally, our system is easier to utilize and exhibits improved flexibility as a tool to detect Cd2+ which belongs to the toxical heavy metal ions.
fig.1. Growth of cells containing smtB-OP-smtA element in LB medium supplemented with 2μM Cd2+. Cells were inoculated at a density of 1x106 cells ml-1, and growth was monitored by measuring the OD540 value. Data points represent the mean values from three separate cultures with SD.
fig.2. A. Metal-induced expression of the pigments (RFP), Rosetta-plysS (1x106 cells ml-1) carrying the smtB-OP-RFP element were grown with Cd2+ and Zn2+ (1-50μM) supplement for 2h immediately before assay and expression was monitored by measuring the OD450 value; B. Metal-induced expression of the pigments (RFP). Rosetta-plysS carrying the smtB-OP-RFP element were grown with Cd2+ and Zn2+ (2μM) supplement for 1-12h immediately before measurement; C. Cadmium-induced expression of the pigment (RFP) at different concentrations. Rosetta-plysS (1x107 cells ml-1) carrying the smtB-OP-RFP element were grown with Cd2+ (1-20μM) supplement for 2h. The data points shown in A and B represent the means of three separate assays with SD.
fig.3. A. Cadmium-induced expression of the pigment (amilCP) at constant concentration. Rosetta-plysS (1x106 cells ml-1) carrying the smtB-OP-amilCP element were grown with Cd2+ (1μM) supplement for 1-2h immediately before assay and the expression was monitored by measuring the OD600 value; B. Cadmium-induced expression of the pigment (amilCP) with different concentrations. Rosetta-plysS carrying the smtB-OP-amilCP device were grown with Cd2+ (1-100μM) supplement for 1h immediately before assay;C. Cadmium-induced expression of the pigment (amilCP) with different concentrations. Rosetta-plysS (1x107 cells ml-1) carrying the smtB-OP-amilCP element were grown with Cd2+ (10, 20, 50, 100, 200 and 500 μM) supplement for 2h. The data points shown in A and B represent the means of three separate values with SD.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]