Difference between revisions of "CRISPR"
m |
|||
| (22 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Catalog/MainLinks}} | ||
| + | |||
<html> | <html> | ||
| + | <table class="collectionTable sortable"> | ||
| + | <tr> | ||
| + | <th style="width: 120px;">Team</th> | ||
| + | <th style="width: 60px;">Year</th> | ||
| + | <th style="width: 60px;">Parts</th> | ||
| + | <th style="width: 160px;">Track</th> | ||
| + | <th>Project Title</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2016.igem.org/Team:NEU-China">NEU-China</a></td> | ||
| + | <td>2016</td> | ||
| + | <td><a target="_blank" href="http://2016.igem.org/Team:NEU-China/Parts">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">ITS COLOUR: A Light-inducible CRISPR/Cas9-mediated gene expression activation system in E. Coli and Yeast</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Aachen">Aachen</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Aachen">Parts</a></td> | ||
| + | <td>Manufacturing</td> | ||
| + | <td class="project">Upcycling Methanol into a Universal Carbon Source</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:BGU_Israel">BGU_Israel</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=BGU_Israel">Parts</a></td> | ||
| + | <td>Health & Medicine</td> | ||
| + | <td class="project">The Boomerang system: engineering logic gate genetic device for detection and treatment of cancer</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:BostonU">BostonU</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=BostonU">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">Developing conditionally dimerizable split protein systems for genetic logic and genome editing applications</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Chalmers-Gothenburg">Chalmers-Gothenburg</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Chalmers-Gothenburg">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">A study in Scarlet</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Hong_Kong_HKU">Hong_Kong_HKU</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Hong_Kong_HKU">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">Controllable cell death and DNA degradation by CRISPR cas system</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:NJAU_China">NJAU_China</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=NJAU_China">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">The Horcrux</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Paris_Bettencourt">Paris_Bettencourt</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Paris_Bettencourt">Parts</a></td> | ||
| + | <td>Food & Nutrition</td> | ||
| + | <td class="project">Ferment It Yourself</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:SCU_China">SCU_China</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=SCU_China">Parts</a></td> | ||
| + | <td>Environment</td> | ||
| + | <td class="project">E. pangu: The Pioneer of Mars</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Stanford-Brown">Stanford-Brown</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Stanford-Brown">Parts</a></td> | ||
| + | <td>Manufacturing</td> | ||
| + | <td class="project">biOrigami: A New Approach to Reduce the Cost of Space Missions</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Tec-Monterrey">Tec-Monterrey</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Tec-Monterrey">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">Insects join iGEM: Sf9 cells as a new chassis for synthetic biology</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Tufts">Tufts</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Tufts">Parts</a></td> | ||
| + | <td>Health & Medicine</td> | ||
| + | <td class="project">Delivery of the CRISPR-Cas9 gene editing platform into epithelial cells using Clostridium difficile toxin B</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Waterloo">Waterloo</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Waterloo">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">CRISPieR: re-engineering CRISPR-Cas9 with functional applications in eukaryotic systems</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Yale">Yale</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Yale">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">Developing a Framework for the Genetic Manipulation of Non-Model and Environmentally Significant Microbes</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:USTC">USTC</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=USTC">Parts</a></td> | ||
| + | <td>Hardware</td> | ||
| + | <td class="project">NDM: Nanomachine Detecting Microbiotics</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Vilnius-Lithuania">Vilnius-Lithuania</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Vilnius-Lithuania">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">Controlling the Lifetime of GMOs using ColiClock</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Duke">Duke</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Duke">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">DNA Sequence Sensing with dCas9 Applied to Antibiotic Resistance Detection and Elimination</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:EPF_Lausanne">EPF_Lausanne</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=EPF_Lausanne">Parts</a></td> | ||
| + | <td>Information Processing</td> | ||
| + | <td class="project">Bio LOGIC: Biologic Orthogonal gRNA-Implemented Circuit</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:NU_Kazakhstan">NU_Kazakhstan</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=NU_Kazakhstan">Parts</a></td> | ||
| + | <td>Health & Medicine</td> | ||
| + | <td class="project">Prevention of Dental Caries by Targeting Streptococcus Mutans </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Peking">Peking</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Peking">Parts</a></td> | ||
| + | <td>Health & Medicine</td> | ||
| + | <td class="project">Fighting Against Tuberculosis: Making Invisible Visible</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Tsinghua">Tsinghua</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Tsinghua">Parts</a></td> | ||
| + | <td>Hardware</td> | ||
| + | <td class="project">Developing light-controlled systems to manipulate genetic information in prokaryotes</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:Washington">Washington</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=Washington">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">Lab on a Strip: Developing a Novel Platform for Yeast Biosensors</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2015.igem.org/Team:William_and_Mary">William_and_Mary</a></td> | ||
| + | <td>2015</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2015&group=William_and_Mary">Parts</a></td> | ||
| + | <td>Measurement</td> | ||
| + | <td class="project">Measurement of Promoter-Based Transcriptional Noise for Application in Gene Network Design</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:British_Columbia">British_Columbia</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=British_Columbia">Parts</a></td> | ||
| + | <td>Food & Energy</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:Chiba">Chiba</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=Chiba">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:Duke">Duke</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=Duke">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:Freiburg">Freiburg</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=Freiburg">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:MIT">MIT</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=MIT">Parts</a></td> | ||
| + | <td>Health & Medicine</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:NJU_NJUT_China">NJU_NJUT_China</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=NJU_NJUT_China">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:Paris_Bettencourt">Paris_Bettencourt</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=Paris_Bettencourt">Parts</a></td> | ||
| + | <td>Health & Medicine</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:Penn_State">Penn_State</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=Penn_State">Parts</a></td> | ||
| + | <td>Manufacturing</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:SJTU-BioX-Shanghai">SJTU-BioX-Shanghai</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=SJTU-BioX-Shanghai">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:Stanford-Brown">Stanford-Brown</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=Stanford-Brown">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:UCSF">UCSF</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=UCSF">Parts</a></td> | ||
| + | <td>Foundational Advance</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><a target="_blank" href="http://2013.igem.org/Team:WHU-China">WHU-China</a></td> | ||
| + | <td>2013</td> | ||
| + | <td><a target="_blank" href="https://parts.igem.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2013&group=WHU-China">Parts</a></td> | ||
| + | <td>New Application</td> | ||
| + | <td class="project">~</td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | </html> | ||
| − | < | + | {{Project/ProjectCSS}} |
| + | <html> | ||
| + | <head> | ||
| − | < | + | <!-- ===Table CSS code:===--> |
| + | <style type="text/css"> | ||
| + | .datagrid table { border-collapse: collapse; text-align: left; width: 100%; } | ||
| + | .datagrid {font: normal 12px/150% Arial, Helvetica, sans-serif; background: #fff; overflow: hidden; border: 1px solid #36752D; -webkit-border-radius: 3px; -moz-border-radius: 3px; border-radius: 3px; } | ||
| + | .datagrid table td, | ||
| + | .datagrid table th { padding: 3px 10px; } | ||
| + | .datagrid table thead th {background:-webkit-gradient( linear, left top, left bottom, color-stop(0.05, #36752D), color-stop(1, #275420) );background:-moz-linear-gradient( center top, #36752D 5%, #275420 100% );filter:progid:DXImageTransform.Microsoft.gradient(startColorstr='#36752D', endColorstr='#275420');background-color:#36752D; color:#FFFFFF; font-size: 15px; font-weight: bold; border-center: 1px solid #36752D; } | ||
| + | .datagrid table thead th:first-child { border: none; } | ||
| + | .datagrid table tbody td { color: #275420; border-left: 1px solid #C6FFC2;font-size: 12px;font-weight: normal; } | ||
| + | .datagrid table tbody .alt td { background: #DFFFDE; color: #275420; } | ||
| + | .datagrid table tbody td:first-child { border-left: none; } | ||
| + | .datagrid table tbody tr:last-child td { border-bottom: none; } | ||
| + | </style> | ||
| + | |||
| + | <h1>Introduction to CRISPR and Cas9</h1> | ||
| + | |||
| + | <img src="https://static.igem.org/mediawiki/2013/1/1b/UBC-CRISPR-Mechanism-Out.png" width="400px" align="right" > | ||
| − | |||
<p> | <p> | ||
| − | + | <strong>From UBC 2013</strong>: CRISPRs (Clustered Regularly Interspaced Short PalindromicRepeats) are specific regions in some bacterial and archaeal genomes that, together with associated Cas (CRISPR-associated) genes, function as an adaptive immune system in prokaryotes. While the specific ‘adaptive’ nature of this immunity is still under investigation, it is known that exogenous DNA is processed by Cas proteins into short (~30 base pair) sequences that are adjacent to the Protospacer Adjacent Motif (PAM) site. These short pieces of DNA are then incorporated into the host genome between repeat sequences to formspacer elements. The repeat-spacer-repeat array is constitutively expressed (pre-CRISPR RNAs or pre-crRNAs) and processed by Cas proteins to form small RNAs (crRNAs). The small RNAs are then loaded into Cas proteins and act to guide them to initiate the sequence-specific cleavage of the target sequence. | |
</p> | </p> | ||
| − | <table> | + | |
| − | <th> | + | |
| − | Team | + | |
| − | </th> | + | |
| − | <th> | + | <p><strong>Background from Freiburg 2013</strong>: Hidden as an uncharacterized <i>E. coli</i> locus for more than 15 years, Barrangou et al. identified the CRISPR (<b>C</b>lusterd <b>R</b>egularly <b>I</b>nterspaced <b>S</b>hort <b>P</b>alindromic <b>R</b>epeats) array as a previously unknown adaptive prokaryotic immune system. Almost half of all prokaryotes make use of this defense mechanism against unselective uptake through natural transformation, phage DNA transduction or horizontal gene transfer by conjugation. Invasive DNA or even RNA can be specifically recognized and efficiently cleaved. This unique feature results from the interaction of non-coding RNAs and CRISPR associated (Cas) proteins. From a wide range of known CRISPR subtypes we used CRISPR type II b of <i>S. pyogenes</i>. |
| − | Track | + | </p> |
| − | </th> | + | |
| − | <th> | + | <p>The recognition and degradation of invasive DNA by CRISPR/Cas type II occurs in three steps: |
| − | Chassis | + | <ol> |
| − | </th> | + | <li><b>Acquisition</b>: Invasive DNA is recognized via a protospacer adjacent motif (PAM) – the sequence NGG. A short sequence downstream of the PAM sequence is then integrated into the host CRISPR array and is termed spacer. Spacer sequences transcribe for CRISPR RNAs(crRNAs) which help to cleave sequence-specific invasive DNA. These sequences are located between short palindromic repeats, which are neccessary for the functionality of the crRNAs.</li> |
| + | |||
| + | <li><b>Expression/Transcription</b>: The Cas9 endonuclease is expressed. CRISPR array is then transcribed and processed by RNAse III into crRNAs. These contain the complementary spacer sequence and the direct repeat sequence. The crRNA guides the Cas9 protein specifically to invasive DNA sequences. Furthermore trans-activating crRNAs (tracrRNA) are transcribed and bind to the direct repeat part of the crRNA. The tracrRNA is necessary for the formation of a Cas9-RNA complex. </li> | ||
| + | |||
| + | <li><b>Interference</b>: Repeatedly invading DNA, which has been integrated into the CRISPR locus, is detected by the RNA-protein complex and cleaved by Cas9.</li> | ||
| + | </ol> | ||
| + | |||
| + | |||
| + | <p> | ||
| + | Each of these teams have worked on CRISPR based systems for at least some part of their projects. Below, you'll find abstracts for each team, direct links to their CRISPR pages and references. Here is the list of 2013 iGEM teams who worked on CRISPR in their projects: | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <div align="center"> | ||
| + | <div style="width:600px;"> | ||
| + | <div class="datagrid"><table> | ||
| + | |||
| + | <thead><th>Team</th> <th>Track</th> <th>Chassis</th></thead> | ||
| + | <tbody> | ||
<tr> | <tr> | ||
<td>British_Columbia</td> <td>Food & Energy</td> <td><i>E. coli</i></td> | <td>British_Columbia</td> <td>Food & Energy</td> <td><i>E. coli</i></td> | ||
| Line 41: | Line 336: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td>Penn_State</td> <td>Manufacturing/td> <td>Plants</td> | + | <td>Penn_State</td> <td>Manufacturing</td> <td>Plants</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 55: | Line 350: | ||
<td>WHU-China</td> <td>New Application</td> <td><i>E. coli</i></td> | <td>WHU-China</td> <td>New Application</td> <td><i>E. coli</i></td> | ||
</tr> | </tr> | ||
| − | </ | + | </tbody> |
| − | + | ||
</table> | </table> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
<h2>CRISPR and Cas9 parts in the Registry</h2> | <h2>CRISPR and Cas9 parts in the Registry</h2> | ||
<p> | <p> | ||
| − | Many of the teams on this page have submitted parts associated with CRISPR | + | Many of the teams on this page have submitted parts associated with CRISPR/Cas9: |
</p> | </p> | ||
| − | + | </html><parttable>CRISPR_Parts</parttable><html> | |
| − | < | + | |
| − | < | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | < | + | <!-- |
| + | <div align="center"> | ||
| + | <div style="width:600px;"> | ||
| + | <div class="datagrid"><table> | ||
| + | <thead><th>iGEM 2013 team</th><th>Part number</th> </thead> | ||
| + | <tbody> | ||
| − | <h2><a href="http://2013.igem.org/Team:British_Columbia">British Columbia</a></h2> | + | <tr> |
| + | <td>UBC</td> <td><a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K1129006">BBa_K1129006</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Freiburg </td><td><a href="https://parts.igem.org/Part:BBa_K1150000">BBa_K1150000</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Freiburg </td><td><a href="https://parts.igem.org/Part:BBa_K1150050">BBa_K1150050</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>MIT </td><td><a href="https://parts.igem.org/Part:BBa_K1179002">BBa_K1179002</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>MIT have many other Cas9 associated parts. Check out the complete list </td><td><a href="http://2013.igem.org/Team:MIT/ResultsBiobricks">here</a>!</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>NJU_NJUT_China </td><td><a href="https://parts.igem.org/Part:BBa_K1160000">BBa_K1160000</a></li> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Paris_Bettencourt </td><td><a href="https://parts.igem.org/Part:BBa_K1137014">BBa_K1137014</a></li> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Paris_Bettencourt (Cas9 recognition sequence) </td><td><a href="https://parts.igem.org/Part:BBa_K1137013">BBa_K1137013</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SJTU-BioX-Shanghai:</td><td><a href="https://parts.igem.org/Part:BBa_K1026000">BBa_K1026000</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SJTU-BioX-Shanghai</td><td><a href="https://parts.igem.org/Part:BBa_K1026001">BBa_K1026001</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SJTU-BioX-Shanghai</td><td><a href="https://parts.igem.org/Part:BBa_K1026002">BBa_K1026002</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Stanford-Brown (<i>E. coli</i> Cas9) </td><td><a href="https://parts.igem.org/Part:BBa_K1218003">BBa_K1218003</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Stanford-Brown (<i>E. coli</i> Cas9) </td><td><a href="https://parts.igem.org/Part:BBa_K1218004">BBa_K1218004</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Stanford-Brown (<i>E. coli</i> Cas9) </td><td><a href="https://parts.igem.org/Part:BBa_K1218011">BBa_K1218011</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>WHU China</td><td><a href="https://parts.igem.org/wiki/index.php?title=Part:BBa_K1081000">BBa_K1081000</a></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | --> | ||
| + | |||
| + | <h2><a href="http://2013.igem.org/Team:British_Columbia">British Columbia 2013</a></h2> | ||
<a href="http://2013.igem.org/Team:British_Columbia/Project/CRISPR">CRISPR used by UBC</a> | <a href="http://2013.igem.org/Team:British_Columbia/Project/CRISPR">CRISPR used by UBC</a> | ||
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:British_Columbia/Project/CRISPR"><img src="https://static.igem.org/mediawiki/2013/1/1b/UBC-CRISPR-Mechanism-Out.png" width="250px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
| − | The past decade has seen the emergence of robust bioprocessing strains engineered to synthesize discrete molecular products. The next-generation of strains could be “programmable,” with on demand generation of molecules within a bioreactor e.g. a yogurt fermentation capable of making any combination of flavouring, nutrients or pharmaceuticals. While merging all this potential into single hosts seems efficient, it would also bring added risk in the case of a process failure due to bacteriophage infection. Here, we not only rationally design widespread immunity to phage infection, but also hack this immunity system to yield programmable biosynthesis at the community level. We demonstrate this by building both broadly and specifically neutralizing CRISPR systems that were paired with biosynthetic capabilities for vanillin, caffeine and cinnamaldehyde production. Eventually, a fermentative process could exist that is vaccinated to phage infection but susceptible to targeted phage addition that results in a programmable probiotic – or ultrabiotic. | + | <strong>Abstract</strong>: The past decade has seen the emergence of robust bioprocessing strains engineered to synthesize discrete molecular products. The next-generation of strains could be “programmable,” with on demand generation of molecules within a bioreactor e.g. a yogurt fermentation capable of making any combination of flavouring, nutrients or pharmaceuticals. While merging all this potential into single hosts seems efficient, it would also bring added risk in the case of a process failure due to bacteriophage infection. Here, we not only rationally design widespread immunity to phage infection, but also hack this immunity system to yield programmable biosynthesis at the community level. We demonstrate this by building both broadly and specifically neutralizing CRISPR systems that were paired with biosynthetic capabilities for vanillin, caffeine and cinnamaldehyde production. Eventually, a fermentative process could exist that is vaccinated to phage infection but susceptible to targeted phage addition that results in a programmable probiotic – or ultrabiotic. |
</p> | </p> | ||
| − | <h2><a href="http://2013.igem.org/Team:Chiba">Chiba</a></h2> | + | <h2><a href="http://2013.igem.org/Team:Chiba">Chiba 2013</a></h2> |
<a href="http://2013.igem.org/Team:Chiba/Parts#CRISPRi">CRISPRi used by the Chiba team</a> | <a href="http://2013.igem.org/Team:Chiba/Parts#CRISPRi">CRISPRi used by the Chiba team</a> | ||
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:Chiba/Parts#CRISPRi"><img src="https://static.igem.org/mediawiki/2013/b/bc/Chiba.CRISPRi.gaiyo.png" width="300px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
| − | In nature, there exist a variety of magnetotactic bacteria. Recently, it was reported that non-magnetotactic cells such as yeast can be magnetized to some extent. We set the goal to transform E. coli into those that are attracted by magnets. By magnetizing E. coli, the cell harvesting process will be much simpler and more economical than the conventional processes such as centrifugation and filtration. To this end, we are conducting three itemized projects. (1) modification of iron transportation network to import as much Fe ions as possible in E. coli, (2) sequestering/ storing iron into human ferritin, and (3) converting cytosolic space from reducing to oxidizing in order to elevate Fe(II)/ Fe(III) ratio within. Because all such manipulations significantly impact the physiology of the host cell, we are establishing the BioBrick platform that enables the temporal knockdown of multiple genes using recently control technology such as CRISPRi. | + | <strong>Abstract</strong>: In nature, there exist a variety of magnetotactic bacteria. Recently, it was reported that non-magnetotactic cells such as yeast can be magnetized to some extent. We set the goal to transform E. coli into those that are attracted by magnets. By magnetizing E. coli, the cell harvesting process will be much simpler and more economical than the conventional processes such as centrifugation and filtration. To this end, we are conducting three itemized projects. (1) modification of iron transportation network to import as much Fe ions as possible in E. coli, (2) sequestering/ storing iron into human ferritin, and (3) converting cytosolic space from reducing to oxidizing in order to elevate Fe(II)/ Fe(III) ratio within. Because all such manipulations significantly impact the physiology of the host cell, we are establishing the BioBrick platform that enables the temporal knockdown of multiple genes using recently control technology such as CRISPRi. |
</p> | </p> | ||
| − | <h2><a href="http://2013.igem.org/Team:Duke">Duke</a></h2> | + | <h2><a href="http://2013.igem.org/Team:Duke">Duke 2013</a></h2> |
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:Duke/Project/Basics"><img src="https://static.igem.org/mediawiki/2013/thumb/9/9f/CRISPR.png/400px-CRISPR.png" width="300px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
| − | Synthetic gene circuits have the potential to revolutionize gene therapies and bio-industrial methods by allowing predictable, customized control of gene expression. Bistable switches and oscillators, key building blocks of more complex gene networks, have been constructed using naturally occurring and well-characterized regulatory elements. In order to expand the versatility and variety of these circuits, we designed and constructed gene networks using artificial transcription factors (ATFs). The ATFs are of two classes: inhibitory TAL proteins and a catalytically inactive dCas9 protein with small guide RNA elements, each orthogonal to the yeast genome. Using mathematical modeling, we determined the parameters expected to create bistability and oscillation, using tandem binding site kinetics to achieve cooperativity. Based on these results, we assembled a library of plasmids containing ATFs, binding sites, regulatory elements, and fluorescent reporters. We then integrated these genes into the genome of Saccharomyces cerevisiae and are currently characterizing them using flow cytometry. | + | <strong>Abstract</strong>: Synthetic gene circuits have the potential to revolutionize gene therapies and bio-industrial methods by allowing predictable, customized control of gene expression. Bistable switches and oscillators, key building blocks of more complex gene networks, have been constructed using naturally occurring and well-characterized regulatory elements. In order to expand the versatility and variety of these circuits, we designed and constructed gene networks using artificial transcription factors (ATFs). The ATFs are of two classes: inhibitory TAL proteins and a catalytically inactive dCas9 protein with small guide RNA elements, each orthogonal to the yeast genome. Using mathematical modeling, we determined the parameters expected to create bistability and oscillation, using tandem binding site kinetics to achieve cooperativity. Based on these results, we assembled a library of plasmids containing ATFs, binding sites, regulatory elements, and fluorescent reporters. We then integrated these genes into the genome of Saccharomyces cerevisiae and are currently characterizing them using flow cytometry. |
<p> | <p> | ||
</p> | </p> | ||
| Line 107: | Line 461: | ||
<ol> | <ol> | ||
<li>Qi L.S, Larson M.H, Gilbert L.A, Doudna J.A, Weissman J.S, Arkin A.P, Lim W.A: Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152:1173-1183.</li> | <li>Qi L.S, Larson M.H, Gilbert L.A, Doudna J.A, Weissman J.S, Arkin A.P, Lim W.A: Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152:1173-1183.</li> | ||
| + | <li>DiCarlo J, Norville J, Mali P, Rios X, Aach J, Church M: Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Research 2013. 41(7), 4336-4343.</li> | ||
</ol> | </ol> | ||
| − | <h2><a href="http://2013.igem.org/Team:Freiburg"> Freiburg</a></h2> | + | <h2><a href="http://2013.igem.org/Team:Freiburg"> Freiburg 2013</a></h2> |
<p> | <p> | ||
| − | + | <a href="http://2013.igem.org/Team:Freiburg/Project/1#background">Freiburg CRISPR page</a> | |
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:Freiburg/Project/1#background"><img src="https://static.igem.org/mediawiki/2013/4/41/UniCAS-toolkit-freiburg-2013.jpg" width="300px"></a> | ||
| + | </div> | ||
| + | |||
</p> | </p> | ||
| + | <p> | ||
| + | <strong>Abstract</strong>: Our Team developed a universal toolkit, termed uniCAS, that enables customizable gene regulation in mammalian cells. Therefore, we engineered the recently discovered and highly promising CRISPR/Cas9 system. The regulation is based on the RNA-guided Cas9 protein, which allows targeting of specific DNA sequences. Our toolkit comprises not only a standardized Cas9 protein, but also different effector domains for efficient gene activation or repression. We further engineered a modular RNA plasmid for easy implementation of RNA guide sequences. As an additional feature, we established an innovative screening method for assessing the functionality of our uniCAS fusion proteins. Single genes and even whole genetic networks can be modified using our uniCAS toolkit. We think that our toolbox of standardized parts of the CRISPR/Cas9 system offers broad application in research fields such as tissue engineering, stem cell reprogramming and fundamental research. | ||
| + | </p> | ||
| + | <p> | ||
| + | Freiburg CRISPR References: | ||
| + | </p> | ||
| + | <ol> | ||
| + | <li> Ishino, Y., <i>et al.</i> (1987). Nucleotide Sequence of the iap Gene in Escherichia coli. Journal of Bacteriology 169, <i>5429-5433</i>. </li> | ||
| + | <li> Barrangou, R., <i>et al.</i> (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, <i>1709-1712</i>. </li> | ||
| + | <li> Marraffini, L., and Sontheimer, E. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, <i>1843-1845</i>.</li> | ||
| + | <li> Jansen, R., <i>et al.</i> (2002). Identification of genes that are associated with DNA repeats in prokaryotes. Molecular Microbiology 43, <i>1565-1575</i>.</li> | ||
| + | <li> Makarova, K., <i>et al.</i> (2011). Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9, <i>467-477</i>.</li> | ||
| + | <li> Jinek, M., <i>et al.</i> (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, <i>816-821</i>.</li> | ||
| + | <li> Cong, L., <i>et al.</i> (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, <i>819-823</i>.</li> | ||
| + | <li> Mali, P., <i>et al.</i> (2013). RNA-guided human genome engineering via Cas9. Science 339, <i>823-826</i>.</li> | ||
| + | <li> Yamanaka, S., <i>et al.</i> (2012). Induced Pluripotent Stem Cells: Past, Present and Future. Cell Stem Cell 10, | ||
| + | <i>678-684</i>.</li> | ||
| + | <li> Kaneshiro, K., <i>et al.</i> (2007). An integrated map of p53-binding sites and histone modification in the human ENCONDE regions. Genomics, | ||
| + | <i>177-188</i>.</li> | ||
| + | </ol> | ||
| − | <h2><a href="http://2013.igem.org/Team:MIT">MIT</a></h2> | + | <h2><a href="http://2013.igem.org/Team:MIT">MIT 2013</a></h2> |
<p> | <p> | ||
<a href="http://2013.igem.org/Team:MIT/Venus">MIT CRISPR page</a></a> | <a href="http://2013.igem.org/Team:MIT/Venus">MIT CRISPR page</a></a> | ||
| + | </p> | ||
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:MIT/Venus"><img src="https://static.igem.org/mediawiki/2013/1/1a/Cas9-splitvenushowitworks.png" width="300px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
| − | Coordinating behavior across cell populations to form synthetic tissues requires spacial communication between individual cells. While there has been some success engineering single signals, sending multiple signaling elements spanning spatial scales for multicellular coordination remains a significant hurdle. Here, we describe a method for mammalian cell-cell communication utilizing engineered exosomes containing miRNA or protein signals. First, we demonstrate selectively packaging signaling miRNAs (miR-451 and miR-503) and synthetic fusion proteins (GFP, Cas9, and Cre recombinase each individually fused to the oligomerizing membrane targeting domain Acyl-TyA) into exosomes within cells engineered with sender genetic circuits. Next, we demonstrate that these miRNA and protein signals can modulate gene expression within cells engineered with receiver genetic circuits. Finally, we present preliminary cell-cell signaling results on populations of cocultured sender and receiver cells. Our method may enable multiplexed communication among populations of various cell types and the creation of sophisticated synthetic tissues. | + | <strong>Abstract</strong>: Coordinating behavior across cell populations to form synthetic tissues requires spacial communication between individual cells. While there has been some success engineering single signals, sending multiple signaling elements spanning spatial scales for multicellular coordination remains a significant hurdle. Here, we describe a method for mammalian cell-cell communication utilizing engineered exosomes containing miRNA or protein signals. First, we demonstrate selectively packaging signaling miRNAs (miR-451 and miR-503) and synthetic fusion proteins (GFP, Cas9, and Cre recombinase each individually fused to the oligomerizing membrane targeting domain Acyl-TyA) into exosomes within cells engineered with sender genetic circuits. Next, we demonstrate that these miRNA and protein signals can modulate gene expression within cells engineered with receiver genetic circuits. Finally, we present preliminary cell-cell signaling results on populations of cocultured sender and receiver cells. Our method may enable multiplexed communication among populations of various cell types and the creation of sophisticated synthetic tissues. |
</p> | </p> | ||
<p> | <p> | ||
| − | + | MIT CRISPR references: | |
</p> | </p> | ||
<ol> | <ol> | ||
| Line 130: | Line 516: | ||
</ol> | </ol> | ||
| − | <h2><a href="http://2013.igem.org/Team:NJU_NJUT_China">NJU NJUT China</a></h2> | + | <h2><a href="http://2013.igem.org/Team:NJU_NJUT_China">NJU NJUT China 2013</a></h2> |
<p> | <p> | ||
| − | Most bacteria and archaea can resist invading DNA and/or RNA elements via the clusters of regularly interspaced short palindromic repeats (CRISPRs).It is believed that the integrated CRISPR sequences have the ability to form a genetic memory which prevents the host from being infected.The memory exist as a DNA library in genome, artificially modified to set its target. The CRISPRs and Cas (CRISPR-associated) interact and form this prokaryotic adaptive immune system. Cas9, as a core of CRISPR system, can play a role of targeted-attacking gene "missiles". Therefore, we build a sort of plasmids, loading CRISPR system, to realize the "killing" of harmful genes and/or organisms. | + | |
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:NJU_NJUT_China/project"><img src="https://static.igem.org/mediawiki/igem.org/d/de/NJU_NJUT_CHINA_Ind1.jpg" width="300px"></a> | ||
| + | </div> | ||
| + | |||
| + | <strong>Abstract</strong>: Most bacteria and archaea can resist invading DNA and/or RNA elements via the clusters of regularly interspaced short palindromic repeats (CRISPRs).It is believed that the integrated CRISPR sequences have the ability to form a genetic memory which prevents the host from being infected.The memory exist as a DNA library in genome, artificially modified to set its target. The CRISPRs and Cas (CRISPR-associated) interact and form this prokaryotic adaptive immune system. Cas9, as a core of CRISPR system, can play a role of targeted-attacking gene "missiles". Therefore, we build a sort of plasmids, loading CRISPR system, to realize the "killing" of harmful genes and/or organisms. | ||
</p> | </p> | ||
<p>CRISPR and Cas9 references:</p> | <p>CRISPR and Cas9 references:</p> | ||
| Line 150: | Line 541: | ||
</ol> | </ol> | ||
| − | <h2><a href="http://2013.igem.org/Team:Paris_Bettencourt">Paris Bettencourt</a></h2> | + | <h2><a href="http://2013.igem.org/Team:Paris_Bettencourt">Paris Bettencourt 2013</a></h2> |
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:Paris_Bettencourt/Project/Detect"><img src="https://static.igem.org/mediawiki/2013/2/2d/PB_13_TB_Sensor_Detect_Overview.png" width="300px"></a> | ||
| + | </div> | ||
<p> | <p> | ||
| − | We are testing new weapons for the global war against Mycobacterium tuberculosis (MTb), a pathogen that infects nearly 2 billion people. Our 4 synergistic projects aim to help in the prevention, diagnosis, and treatment of tuberculosis. 1) We are reproducing an essential MTb metabolic pathway in E. coli, where it can be easily and safely targeted in a drug screen. 2) We are building a phage-based biosensor to allow the rapid diagnosis specifically drug-resistant MTb strains. 3) We are constructing a mycobacteriophage to detect and counterselect drug-resistant Mtb in the environment. 4) We are programming E. coli to follow MTb into human macrophages and saturate it with bacteriolytic enzymes. We want to vanquish tuberculosis and build a TB-free world. | + | <strong>Abstract</strong>: We are testing new weapons for the global war against Mycobacterium tuberculosis (MTb), a pathogen that infects nearly 2 billion people. Our 4 synergistic projects aim to help in the prevention, diagnosis, and treatment of tuberculosis. 1) We are reproducing an essential MTb metabolic pathway in E. coli, where it can be easily and safely targeted in a drug screen. 2) We are building a phage-based biosensor to allow the rapid diagnosis specifically drug-resistant MTb strains. 3) We are constructing a mycobacteriophage to detect and counterselect drug-resistant Mtb in the environment. 4) We are programming E. coli to follow MTb into human macrophages and saturate it with bacteriolytic enzymes. We want to vanquish tuberculosis and build a TB-free world. |
</p> | </p> | ||
| − | <h2><a href="http://2013.igem.org/Team:Penn_State">Penn State</a></h2> | + | <h2><a href="http://2013.igem.org/Team:Penn_State">Penn State 2013</a></h2> |
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:Penn_State/Cas9Project"><img src="http://cfile204.uf.daum.net/R320x0/253C363DA4B1DC52189C6F" width="300px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
<a href="http://2013.igem.org/Team:Penn_State/Cas9Project">Penn State plan Cas9 project information </a> | <a href="http://2013.igem.org/Team:Penn_State/Cas9Project">Penn State plan Cas9 project information </a> | ||
</p> | </p> | ||
<p> | <p> | ||
| − | Plants as Plants: Natural Factories provides a green approach to the manufacturing of valuable chemicals and materials. Through synthetic biology, we are able to control the expression of genes that regulate the production of desired secondary metabolites. Via the manipulation of established metabolic pathways, we hope to produce vanillin and butanol. The prospect of being able to synthetically produce a biofuel provides vast possibilities for the scope of synthetic biology and green energy. Additionally through the manipulation of the cellulose synthase genes, we hope to increase the biomass of plants by a hybrid plant cell wall. As shown through these projects, the use of plants provides various green energy possibilities. However, due to the limited use of plants within synthetic biology there are various regulation issues. Thus we have additionally worked on characterizing a range of plant promoters as well as introducing the Cas9 crisper system into plants. | + | <strong>Abstract</strong>: Plants as Plants: Natural Factories provides a green approach to the manufacturing of valuable chemicals and materials. Through synthetic biology, we are able to control the expression of genes that regulate the production of desired secondary metabolites. Via the manipulation of established metabolic pathways, we hope to produce vanillin and butanol. The prospect of being able to synthetically produce a biofuel provides vast possibilities for the scope of synthetic biology and green energy. Additionally through the manipulation of the cellulose synthase genes, we hope to increase the biomass of plants by a hybrid plant cell wall. As shown through these projects, the use of plants provides various green energy possibilities. However, due to the limited use of plants within synthetic biology there are various regulation issues. Thus we have additionally worked on characterizing a range of plant promoters as well as introducing the Cas9 crisper system into plants. |
</p> | </p> | ||
| − | <h2><a href="http://2013.igem.org/Team:SJTU-BioX-Shanghai">SJTU BioX Shanghai</a></h2> | + | <br> |
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | <h2><a href="http://2013.igem.org/Team:SJTU-BioX-Shanghai">SJTU BioX Shanghai 2013</a></h2> | ||
<p> | <p> | ||
<a href="http://2013.igem.org/Team:SJTU-BioX-Shanghai/Project/CRISPRi/Introdution">SJTU-BioX-Shanghai CRISPR page</a> | <a href="http://2013.igem.org/Team:SJTU-BioX-Shanghai/Project/CRISPRi/Introdution">SJTU-BioX-Shanghai CRISPR page</a> | ||
</p> | </p> | ||
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:SJTU-BioX-Shanghai/Project/CRISPRi/Introdution"><img src="https://static.igem.org/mediawiki/2013/thumb/0/06/CRISPRi.png/250px-CRISPRi.png" width="300px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
| − | Few researches have been done to regulate gene expression levels in genomic scale so far. This year we aim to combine two systems together in order to provide a universal and convenient tool which can be used to regulate different genomic genes simultaneously and independently in a quantitative way. Our project involves the newly developed gene regulating tool CRISPRi and three light-controlled expression systems induced by red, green, and blue light respectively. Simply by changing the regulating parts in CRISPRi system towards mRFP, luciferase, and three enzymes, we hope to prove our system can be used qualitatively, quantitatively and practically step by step. We have also designed a box and written a software as our experiment measurements. Simply by typing in several parameters, different gene expression levels can be controlled. This system can also be improved to predict the maximized producing efficiency after some simple tests in future. | + | <strong>Abstract</strong>: Few researches have been done to regulate gene expression levels in genomic scale so far. This year we aim to combine two systems together in order to provide a universal and convenient tool which can be used to regulate different genomic genes simultaneously and independently in a quantitative way. Our project involves the newly developed gene regulating tool CRISPRi and three light-controlled expression systems induced by red, green, and blue light respectively. Simply by changing the regulating parts in CRISPRi system towards mRFP, luciferase, and three enzymes, we hope to prove our system can be used qualitatively, quantitatively and practically step by step. We have also designed a box and written a software as our experiment measurements. Simply by typing in several parameters, different gene expression levels can be controlled. This system can also be improved to predict the maximized producing efficiency after some simple tests in future. |
<p> | <p> | ||
SJTU-BioX-Shanghai CRISPR references: | SJTU-BioX-Shanghai CRISPR references: | ||
| Line 180: | Line 588: | ||
</p> | </p> | ||
| − | <h2><a href="http://2013.igem.org/Team:Stanford-Brown">Stanford Brown</a></h2> | + | <h2><a href="http://2013.igem.org/Team:Stanford-Brown">Stanford Brown 2013</a></h2> |
| + | |||
<p> | <p> | ||
<a href="http://2013.igem.org/Team:Stanford-Brown/Projects/CRISPR">Stanford-Brown CRISPR page</a> | <a href="http://2013.igem.org/Team:Stanford-Brown/Projects/CRISPR">Stanford-Brown CRISPR page</a> | ||
</p> | </p> | ||
<p> | <p> | ||
| − | Communication is a dynamic requirement for life as we know it. We are using cellular and molecular messaging of different magnitudes to improve the broadcasting and reception of information. Starting on the atomic level, our BioWires project has created silver-incorporating DNA to act as nanowires, which could improve the cost and effectiveness of electronic products. Our CRISPR project is creating a system for DNA messages and resistances to be passed from cell to cell, in effect, creating transmissible probiotics and changing the way that cells communicate. We are also building a chromogenic biosensor to detect sucrose secretion that will be launched on a satellite (EuCROPIS) into low-Earth orbit. Finally, our De-Extinction project involves decoding messages from the past to better understand early life on Earth. | + | <strong>Abstract</strong>: Communication is a dynamic requirement for life as we know it. We are using cellular and molecular messaging of different magnitudes to improve the broadcasting and reception of information. Starting on the atomic level, our BioWires project has created silver-incorporating DNA to act as nanowires, which could improve the cost and effectiveness of electronic products. Our CRISPR project is creating a system for DNA messages and resistances to be passed from cell to cell, in effect, creating transmissible probiotics and changing the way that cells communicate. We are also building a chromogenic biosensor to detect sucrose secretion that will be launched on a satellite (EuCROPIS) into low-Earth orbit. Finally, our De-Extinction project involves decoding messages from the past to better understand early life on Earth. |
</p> | </p> | ||
<p> | <p> | ||
| Line 202: | Line 611: | ||
</ol> | </ol> | ||
| − | <h2><a href="http://2013.igem.org/Team:UCSF">UCSF</a></h2> | + | <h2><a href="http://2013.igem.org/Team:UCSF">UCSF 2013</a></h2> |
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:UCSF/Project/Background"><img src="https://static.igem.org/mediawiki/2013/5/56/CRISPRi_Background_UCSF.png" width="300px"></a> | ||
| + | </div> | ||
| + | |||
<p> | <p> | ||
<a href="http://2013.igem.org/Team:UCSF/Project/Background">UCSF CRISPR page</a> | <a href="http://2013.igem.org/Team:UCSF/Project/Background">UCSF CRISPR page</a> | ||
</p> | </p> | ||
<p> | <p> | ||
| − | In microbial communities, bacterial populations are commonly controlled using indiscriminate, broad range antibiotics. There are few ways to target specific strains effectively without disrupting the entire microbiome and local environment. The goal of our project is to take advantage of a natural horizontal gene transfer mechanism in bacteria to precisely affect gene expression in selected strains. We combine bacterial conjugation with CRISPRi, an RNAi-like repression system developed from bacteria, to regulate gene expression in targeted strains within a complex microbial community. One possible application is to selectively repress pathogenic genes in a microbiome, leaving the community makeup unaffected. In addition, we use CRISPRi to lay the groundwork for transferring large circuits that enable complex functionality and decision-making in cells. | + | <strong>Abstract</strong>: In microbial communities, bacterial populations are commonly controlled using indiscriminate, broad range antibiotics. There are few ways to target specific strains effectively without disrupting the entire microbiome and local environment. The goal of our project is to take advantage of a natural horizontal gene transfer mechanism in bacteria to precisely affect gene expression in selected strains. We combine bacterial conjugation with CRISPRi, an RNAi-like repression system developed from bacteria, to regulate gene expression in targeted strains within a complex microbial community. One possible application is to selectively repress pathogenic genes in a microbiome, leaving the community makeup unaffected. In addition, we use CRISPRi to lay the groundwork for transferring large circuits that enable complex functionality and decision-making in cells. |
</p> | </p> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | <h2><a href="http://2013.igem.org/Team:WHU-China">WHU China 2013</a></h2> | ||
| + | |||
| + | <div class = "aluno"> | ||
| + | <a href="http://2013.igem.org/Team:WHU-China/background#CRISPR"><img src="https://static.igem.org/mediawiki/2013/7/74/WHUCas9.png" width="300px"></a> | ||
| + | </div> | ||
| − | |||
<p> | <p> | ||
<a href="http://2013.igem.org/Team:WHU-China/background#CRISPR">WHU-China CRISPR page</a> | <a href="http://2013.igem.org/Team:WHU-China/background#CRISPR">WHU-China CRISPR page</a> | ||
</p> | </p> | ||
<p> | <p> | ||
| − | Cas9 is an RNA-guided dsDNA nuclease utilized by bacteria immune system. The genetically engineered Cas9 has recently been shown to have the ability to repress or activate desired gene expression. | + | <strong>Abstract</strong>: Cas9 is an RNA-guided dsDNA nuclease utilized by bacteria immune system. The genetically engineered Cas9 has recently been shown to have the ability to repress or activate desired gene expression. In practical research and industrial application, we usually face the problem to express a gene at different levels, not only “on” or “off ”, so a more flexible regulation method is needed. To achieve multi-stage regulation of target genes, we further develop several dCas9 devices in which dCas9 alone or fused with omega subunit of RNAP is directed by various guide RNAs to different regions of designed double promoters. Therefore, promoters with disparate strength can be either activated or repressed respectively and multi-stage gene expression can be achieved. Also, based on such novel technology platform, we are developing diverse applications such as a guide RNA-mediated oscillator. |
| − | In practical research and industrial application, we usually face the problem to express a gene at different levels, not only “on” or “off ”, so a more flexible regulation method is needed. To achieve multi-stage regulation of target genes, we further develop several dCas9 devices in which dCas9 alone or fused with omega subunit of RNAP is directed by various guide RNAs to different regions of designed double promoters. Therefore, promoters with disparate strength can be either activated or repressed respectively and multi-stage gene expression can be achieved. Also, based on such novel technology platform, we are developing diverse applications such as a guide RNA-mediated oscillator. | + | |
</p> | </p> | ||
<p> | <p> | ||
Latest revision as of 15:52, 16 July 2020
| Team | Year | Parts | Track | Project Title |
|---|---|---|---|---|
| NEU-China | 2016 | Parts | New Application | ITS COLOUR: A Light-inducible CRISPR/Cas9-mediated gene expression activation system in E. Coli and Yeast |
| Aachen | 2015 | Parts | Manufacturing | Upcycling Methanol into a Universal Carbon Source |
| BGU_Israel | 2015 | Parts | Health & Medicine | The Boomerang system: engineering logic gate genetic device for detection and treatment of cancer |
| BostonU | 2015 | Parts | Foundational Advance | Developing conditionally dimerizable split protein systems for genetic logic and genome editing applications |
| Chalmers-Gothenburg | 2015 | Parts | New Application | A study in Scarlet |
| Hong_Kong_HKU | 2015 | Parts | New Application | Controllable cell death and DNA degradation by CRISPR cas system |
| NJAU_China | 2015 | Parts | New Application | The Horcrux |
| Paris_Bettencourt | 2015 | Parts | Food & Nutrition | Ferment It Yourself |
| SCU_China | 2015 | Parts | Environment | E. pangu: The Pioneer of Mars |
| Stanford-Brown | 2015 | Parts | Manufacturing | biOrigami: A New Approach to Reduce the Cost of Space Missions |
| Tec-Monterrey | 2015 | Parts | New Application | Insects join iGEM: Sf9 cells as a new chassis for synthetic biology |
| Tufts | 2015 | Parts | Health & Medicine | Delivery of the CRISPR-Cas9 gene editing platform into epithelial cells using Clostridium difficile toxin B |
| Waterloo | 2015 | Parts | Foundational Advance | CRISPieR: re-engineering CRISPR-Cas9 with functional applications in eukaryotic systems |
| Yale | 2015 | Parts | Foundational Advance | Developing a Framework for the Genetic Manipulation of Non-Model and Environmentally Significant Microbes |
| USTC | 2015 | Parts | Hardware | NDM: Nanomachine Detecting Microbiotics |
| Vilnius-Lithuania | 2015 | Parts | Foundational Advance | Controlling the Lifetime of GMOs using ColiClock |
| Duke | 2015 | Parts | Foundational Advance | DNA Sequence Sensing with dCas9 Applied to Antibiotic Resistance Detection and Elimination |
| EPF_Lausanne | 2015 | Parts | Information Processing | Bio LOGIC: Biologic Orthogonal gRNA-Implemented Circuit |
| NU_Kazakhstan | 2015 | Parts | Health & Medicine | Prevention of Dental Caries by Targeting Streptococcus Mutans |
| Peking | 2015 | Parts | Health & Medicine | Fighting Against Tuberculosis: Making Invisible Visible |
| Tsinghua | 2015 | Parts | Hardware | Developing light-controlled systems to manipulate genetic information in prokaryotes |
| Washington | 2015 | Parts | New Application | Lab on a Strip: Developing a Novel Platform for Yeast Biosensors |
| William_and_Mary | 2015 | Parts | Measurement | Measurement of Promoter-Based Transcriptional Noise for Application in Gene Network Design |
| British_Columbia | 2013 | Parts | Food & Energy | ~ |
| Chiba | 2013 | Parts | New Application | ~ |
| Duke | 2013 | Parts | New Application | ~ |
| Freiburg | 2013 | Parts | Foundational Advance | ~ |
| MIT | 2013 | Parts | Health & Medicine | ~ |
| NJU_NJUT_China | 2013 | Parts | New Application | ~ |
| Paris_Bettencourt | 2013 | Parts | Health & Medicine | ~ |
| Penn_State | 2013 | Parts | Manufacturing | ~ |
| SJTU-BioX-Shanghai | 2013 | Parts | New Application | ~ |
| Stanford-Brown | 2013 | Parts | New Application | ~ |
| UCSF | 2013 | Parts | Foundational Advance | ~ |
| WHU-China | 2013 | Parts | New Application | ~ |
Introduction to CRISPR and Cas9

From UBC 2013: CRISPRs (Clustered Regularly Interspaced Short PalindromicRepeats) are specific regions in some bacterial and archaeal genomes that, together with associated Cas (CRISPR-associated) genes, function as an adaptive immune system in prokaryotes. While the specific ‘adaptive’ nature of this immunity is still under investigation, it is known that exogenous DNA is processed by Cas proteins into short (~30 base pair) sequences that are adjacent to the Protospacer Adjacent Motif (PAM) site. These short pieces of DNA are then incorporated into the host genome between repeat sequences to formspacer elements. The repeat-spacer-repeat array is constitutively expressed (pre-CRISPR RNAs or pre-crRNAs) and processed by Cas proteins to form small RNAs (crRNAs). The small RNAs are then loaded into Cas proteins and act to guide them to initiate the sequence-specific cleavage of the target sequence.
Background from Freiburg 2013: Hidden as an uncharacterized E. coli locus for more than 15 years, Barrangou et al. identified the CRISPR (Clusterd Regularly Interspaced Short Palindromic Repeats) array as a previously unknown adaptive prokaryotic immune system. Almost half of all prokaryotes make use of this defense mechanism against unselective uptake through natural transformation, phage DNA transduction or horizontal gene transfer by conjugation. Invasive DNA or even RNA can be specifically recognized and efficiently cleaved. This unique feature results from the interaction of non-coding RNAs and CRISPR associated (Cas) proteins. From a wide range of known CRISPR subtypes we used CRISPR type II b of S. pyogenes.
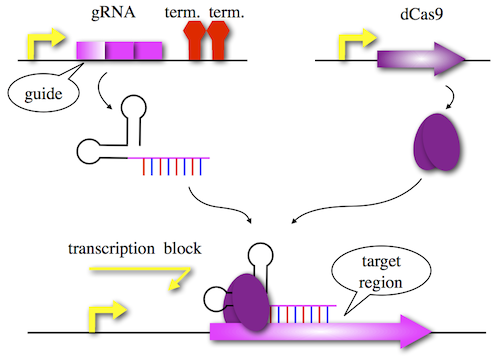
The recognition and degradation of invasive DNA by CRISPR/Cas type II occurs in three steps:
- Acquisition: Invasive DNA is recognized via a protospacer adjacent motif (PAM) – the sequence NGG. A short sequence downstream of the PAM sequence is then integrated into the host CRISPR array and is termed spacer. Spacer sequences transcribe for CRISPR RNAs(crRNAs) which help to cleave sequence-specific invasive DNA. These sequences are located between short palindromic repeats, which are neccessary for the functionality of the crRNAs.
- Expression/Transcription: The Cas9 endonuclease is expressed. CRISPR array is then transcribed and processed by RNAse III into crRNAs. These contain the complementary spacer sequence and the direct repeat sequence. The crRNA guides the Cas9 protein specifically to invasive DNA sequences. Furthermore trans-activating crRNAs (tracrRNA) are transcribed and bind to the direct repeat part of the crRNA. The tracrRNA is necessary for the formation of a Cas9-RNA complex.
- Interference: Repeatedly invading DNA, which has been integrated into the CRISPR locus, is detected by the RNA-protein complex and cleaved by Cas9.
Each of these teams have worked on CRISPR based systems for at least some part of their projects. Below, you'll find abstracts for each team, direct links to their CRISPR pages and references. Here is the list of 2013 iGEM teams who worked on CRISPR in their projects:
| Team | Track | Chassis |
|---|---|---|
| British_Columbia | Food & Energy | E. coli |
| Chiba | New Application | E. coli |
| Duke | New Application | Yeast / S. cerevisiae |
| Freiburg | Foundational Advance | E. coli / Mammalian |
| MIT | Health & Medicine | Mammalian |
| NJU_NJUT_China | New Application | E. coli |
| Paris_Bettencourt | Health & Medicine | E. coli |
| Penn_State | Manufacturing | Plants |
| SJTU-BioX-Shanghai | New Application | E. coli |
| Stanford-Brown | New Application | E. coli / B. subtilis |
| UCSF | Foundational Advance | E. coli |
| WHU-China | New Application | E. coli |
CRISPR and Cas9 parts in the Registry
Many of the teams on this page have submitted parts associated with CRISPR/Cas9:
| Name | Description | Type | Created by | length | uses | seq |
|---|---|---|---|---|---|---|
| BBa_K1218003 | CRISPR CasA E. coli (Modern) | Coding | Trevor Kalkus, Gordon Wade, Alissa Greenberg | 1509 | . . . aaaccgcaaggagggccatcaaatggctga | |
| BBa_K1218011 | Cas9 | Coding | Sophia Liang | 5080 | 2 | . . . catgttataataggcaaaagaagagtagtg |
| BBa_K1129006 | Cas 9 from Streptococcus thermophilus | Coding | UBC iGEM 2013 | 4167 | . . . atagaccttgccaaactaggagagggttaa | |
| BBa_K1026002 | Constitutively Expressed gRNA targeting mRFP | Composite | Hongyi WU | 266 | . . . caccttcgggtgggcctttctgcgtttata | |
| BBa_K1218004 | CRISPR CasA (Ancestral) | Coding | Trevor Kalkus, Gordon Wade, Alissa Greenberg | 1340 | . . . gatcacaaccaccgtcataagcattaatga | |
| BBa_K1081000 | J13002-dcas9 | Composite | Hangxing Jia | 4180 | . . . cgcattgatttgagtcagctaggaggtgac | |
| BBa_K1160000 | coding sequence of Cas9 from CRISPR system type II | Plasmid | Huang Xingxu | 9159 | . . . acatttccccgaaaagtgccacctgacgtc | |
| BBa_K1150000 | dCas9 | Coding | Freiburg 2013 | 4101 | 42 | . . . cggatcgacctgtctcagctgggaggcgac |
| BBa_K1150017 | dCas9 with CMV promoter | Device | Freiburg 2013 | 5012 | . . . aggcatgctggggatgcggtgggctctatg | |
| BBa_K1026000 | Constitutively Expressed dCas9 Operon | Composite | Hongyi WU | 4311 | . . . caccttcgggtgggcctttctgcgtttata | |
| BBa_K1150050 | Truncated CMV dCas9 Device #4 | Device | Natalie Louis and Lisa Schmunk | 3626 | . . . aggcatgctggggatgcggtgggctctatg | |
| BBa_K1179002 | Hef1A_Cas9-VP16 | Generator | Brandon Nadres | 5141 | . . . ggtgggacgcgtgagcttcagtgcaggtga | |
| BBa_K1137013 | crRNA anti KAN | Coding | Nicolas Koutsoubelis, Anne Loechner | 251 | . . . aaacttcagcacactgagacttgttgagtt | |
| BBa_K1982000 | tCas9-CIBN (Prokaryotic LACE system) | Device | Zexu Li | 4731 | . . . ccatacgatgttccagattacgcttaataa | |
| BBa_K1982001 | Prokaryotic tCAS9 | Coding | Zexu Li | 4122 | . . . aggattgacctgtcccaactgggaggcgac | |
| BBa_K1982002 | Prokaryotic Cryptochrome 2 (CRY2) ( a blue light stimulated photoreceptor) | Coding | Zexu Li | 1854 | . . . actacaagtttgggaaaaaatggttgcaaa | |
| BBa_K1982003 | CIBN(the N-terminal fragment of CIB1) | Coding | Zexu Li | 612 | . . . ccatacgatgttccagattacgcttaataa | |
| BBa_K1982004 | tCas9-CIBN (Prokaryotic LACE system) | Device | Zexu Li | 4731 | . . . ccatacgatgttccagattacgcttaataa | |
| BBa_K1982005 | CRY2-VP64(Prokaryotic LACE system) | Device | Zexu Li | 2100 | . . . gactacaaggacgacgacgacaaataataa | |
| BBa_K1982006 | tCas9-Vp64(Prokaryotic) | Device | Zexu Li | 4368 | . . . gactacaaggacgacgacgacaaataataa | |
| BBa_K1994013 | sgRNA with dCas9 binding site sequence 9 and PP7 handle insert | RNA | Liam Carroll | 189 | . . . gcacgtcatctgacgtgccttttttattta | |
| BBa_K1994017 | sgRNA with 5' golden gate adapter and PP7 protein binding site | RNA | Liam Carroll | 178 | . . . ggcacgtcatctgacgtgccttttttattt | |
| BBa_K2017000 | C-split Cas9 + DnaE C-intein | Protein_Domain | Monica Victoria Gutierrez Salazar | 2358 | . . . aaggtgcccaagaagaagaggaaggtgtga | |
| BBa_K2017001 | N-split Cas9 + DnaE N-intein | Protein_Domain | Monica Victoria Gutierrez Salazar | 2241 | . . . gatttgatgagggtggacaacctccctaac | |
| BBa_K2017007 | 35s:5'+ Ga20ox consense + SAGTI-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 3057 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017008 | 35s + Ga20ox consense + RSIAT-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 2836 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017009 | 35s + Ga20ox consense + AEK-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 2872 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017011 | 35s:5' + TFL consense + SAGTI-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 3057 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017010 | 35s + Ga20ox consense + RSIAT-TEV-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 2869 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017012 | 35s + TFL consense + RSIAT-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 2836 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017013 | 35s + TFL consense + AEK-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 2872 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K2017014 | 35s + TFL consense + RSIAT-TEV-Luciferase + Tnos | Device | Monica Victoria Gutierrez Salazar | 2869 | . . . gttactagatcggcaattccgctagagacc | |
| BBa_K1982007 | Eukaryotic tCAS9 | Coding | Zexu Li | 4121 | . . . aaggattgacctgtcccaactgggaggcga | |
| BBa_K1982008 | tCas9-CIBN (Eukaryotic LACE system) | Coding | Zexu Li | 4731 | . . . ccatacgatgttccagattacgcttaataa | |
| BBa_K1982010 | CRY2-VP64(Eukaryotic LACE system) | Coding | Zexu Li | 2100 | . . . gactacaaggacgacgacgacaaataataa | |
| BBa_K1982011 | tCas9-Vp64(Eukaryoticc) | Coding | Zexu Li | 4368 | . . . gactacaaggacgacgacgacaaataataa | |
| BBa_K1946002 | sgRNA targeting LacI | RNA | Musa Efe Işılak | 205 | . . . tctgatgagtccgtgaggacgaaaaaaaaa | |
| BBa_K1994021 | sgRNA containing two golden gate adapters | RNA | Isobel Holden | 157 | . . . ggcacgtcatctgacgtgccttttttattt | |
| BBa_K1994025 | BsaI-GFP-dCas9 | Composite | Egheosa Ogbomo | 5075 | . . . attgatttgagtcagctaggaggtgactga | |
| BBa_K2483005 | sgRNA target site couples facing each other with 6 bp spacer | DNA | Sophia Borowski | 850 | . . . cgtctgtaatcgccctttgtacgtgaacgg | |
| BBa_K2483006 | sgRNA target site couples facing each other with 18 bp spacer | DNA | Bryan Nowack | 955 | . . . ttgaccgcggtcttcctccacattcctgtc | |
| BBa_K2361000 | spdCas9 | Coding | Mart Bartelds | 4120 | . . . cagctaggaggtgactaagtcgacctcgag | |
| BBa_K2361001 | dCas9 VRER | Coding | Mart Bartelds | 4108 | . . . attgatttgagtcagctaggaggtgactga | |
| BBa_K2361004 | CRISPR array | RNA | Sebald Verkuijl | 639 | . . . tttatctgttgtttgtcggtgaacgctctc | |
| BBa_K2371004 | sgRNA generator for EML4-ALK variant A 23 | Composite | Qi Xiao | 164 | . . . gcctctaaacgggtcttgaggggttttttg | |
| BBa_K2371005 | sgRNA generator for EML4-ALK variant A 33 | Composite | Qi Xiao | 164 | . . . gcctctaaacgggtcttgaggggttttttg | |
| BBa_K2371006 | sgRNA generator for EML4-ALK variant A 83 | Composite | Qi Xiao | 164 | . . . gcctctaaacgggtcttgaggggttttttg | |
| BBa_K2558201 | dCas9 generator with Anderson weak promotor | Generator | Tianze Huang | 4308 | . . . caccttcgggtgggcctttctgcgtttata | |
| BBa_K2558003 | dCas9 | Coding | Tianze Huang | 4107 | 6 | . . . attgatttgagtcagctaggaggtgactaa |
| BBa_K2627003 | crRNA targeting GltA | RNA | Zhaoqin Zhang | 162 | . . . tttttttaagcttggctgttttggcggatg | |
| BBa_K2627004 | crRNA targeting GltA | Composite | Zhaoqin Zhang | 477 | . . . ggatttgaacgttgcgaaggatagcaccac | |
| BBa_K2660006 | N-Cas9 | Coding | Victor Nunes de Jesus, Danielle Biscaro Pedrolli | 3459 | . . . ctagtggttgctaaggtggaaaaagggaaa | |
| BBa_K2660007 | C-Cas9 | Coding | Danielle Biscaro Pedrolli,Victor Nunes de Jesus | 648 | . . . attgatttgagtcagcttggcggtgactga | |
| BBa_K3799000 | EiCsm6,A CRISPR system endoribonuclease | Coding | Shubhamay Das | 1293 | -1 | . . . ttcaaccaatccatcaaggagctgctttaa |
| BBa_K3791000 | Spacer gRNA Ampicillin | DNA | Auba Fuster Pal�, Laura S�nchez Ruiz | 20 | -1 | tccgcctccatccagtctat |
| BBa_K3977000 | Cas12j-D394A (or dCasΦ), for more see 2021 SCU-China wiki page result and model | Coding | Yilong Xu | 2271 | -1 | . . . accccggctcaggaaccgtcccagactagc |
| BBa_K3791001 | Spacer gRNA Chloramphenicol | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 20 | -1 | tgatggcttccatgtcggca |
| BBa_K3791002 | Spacer gRNA Erythromycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 20 | -1 | cgcagcagagaagcctggat |
| BBa_K3791003 | Spacer gRNA Kanamycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 20 | -1 | caccatgatattcggcaagc |
| BBa_K3791004 | Spacer gRNA Spectinomycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 20 | -1 | gctcatcgccagcccagtcg |
| BBa_K3791005 | gRNA Ampicillin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 41 | -1 | . . . aagtgtagattccgcctccatccagtctat |
| BBa_K3791006 | gRNA Chloramphenicol | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 41 | -1 | . . . aagtgtagattgatggcttccatgtcggca |
| BBa_K3791007 | gRNA Erythromycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 41 | -1 | . . . aagtgtagatcgcagcagagaagcctggat |
| BBa_K3791008 | gRNA Kanamycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 41 | -1 | . . . aagtgtagatcaccatgatattcggcaagc |
| BBa_K3791009 | gRNA Spectinomycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 41 | -1 | . . . aagtgtagatgctcatcgccagcccagtcg |
| BBa_K3791010 | gRNA Ampicillin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 59 | -1 | . . . aagtgtagattccgcctccatccagtctat |
| BBa_K3791011 | gRNA Chloramphenicol construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 59 | -1 | . . . aagtgtagattgatggcttccatgtcggca |
| BBa_K3791012 | gRNA Erythromycin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 59 | -1 | . . . aagtgtagatcgcagcagagaagcctggat |
| BBa_K3791013 | gRNA Kanamycin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 59 | -1 | . . . aagtgtagatcaccatgatattcggcaagc |
| BBa_K3791014 | gRNA Spectinomycin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 59 | -1 | . . . aagtgtagatgctcatcgccagcccagtcg |
| BBa_K3791015 | Efficient gRNA Ampicillin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 66 | -1 | . . . tctattaattaatttctactaagtgtagat |
| BBa_K3791016 | Efficient gRNA Chloramphenicol | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 66 | -1 | . . . cggcagaattaatttctactaagtgtagat |
| BBa_K3791017 | Efficient gRNA Erythromycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 66 | -1 | . . . tggatgttataatttctactaagtgtagat |
| BBa_K3791018 | Efficient gRNA Kanamycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 66 | -1 | . . . caagcaggctaatttctactaagtgtagat |
| BBa_K3791019 | Efficient gRNA Spectinomycin | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 66 | -1 | . . . agtcgggcgtaatttctactaagtgtagat |
| BBa_K3791020 | Efficient gRNA Ampicillin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 145 | -1 | . . . cgaaaggggggccttttttcgttttggtcc |
| BBa_K3791021 | Efficient gRNA Chloramphenicol construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 145 | -1 | . . . cgaaaggggggccttttttcgttttggtcc |
| BBa_K3791022 | Efficient gRNA Erythromycin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 145 | -1 | . . . cgaaaggggggccttttttcgttttggtcc |
| BBa_K3791023 | Efficient gRNA Kanamycin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 145 | -1 | . . . cgaaaggggggccttttttcgttttggtcc |
| BBa_K3791024 | Efficient gRNA Spectinomycin construct | DNA | Laura S�nchez Ruiz, Auba Fuster Pal� | 145 | -1 | . . . cgaaaggggggccttttttcgttttggtcc |
| BBa_K4298001 | yEvolvr-TM8.3 | Composite | Buyao Li | 7802 | -1 | . . . caacttgaaaaagtggcaccgagtcggtgc |
| BBa_K4298002 | enCas9-PolI5M | Coding | Buyao Li | 7698 | -1 | . . . gttttgggacgctcgaaggctttaatttgc |
| BBa_K5490001 | 23-nt sequence binds CasRx to cleave WNV genome; modifiable target 2 | RNA | IOANNIS VASILEIOS ELAFROPOULOS | 33 | -1 | . . . cgaagaacgccaagagagccaacacaaaac |
| BBa_K5490008 | Scaffold or direct repeat region (DR 36) | Regulatory | IOANNIS VASILEIOS ELAFROPOULOS | 36 | -1 | . . . aacccctaccaactggtcggggtttgaaac |
| BBa_K5490009 | Scaffold or direct repeat region (DR 30) | Regulatory | IOANNIS VASILEIOS ELAFROPOULOS | 30 | -1 | aacccctaccaactggtcggggtttgaaac |
| BBa_K5490018 | gRNA FOR CASRX , SPACER 1 (WNV) | RNA | IOANNIS VASILEIOS ELAFROPOULOS | 99 | -1 | . . . aacccctaccaactggtcggggtttgaaac |
| BBa_K5490019 | gRNA FOR CASRX , SPACER 2 (WNV) | RNA | IOANNIS VASILEIOS ELAFROPOULOS | 99 | -1 | . . . aacccctaccaactggtcggggtttgaaac |
| BBa_K5490020 | gRNA FOR CASRX , SPACER 3 (WNV) | RNA | IOANNIS VASILEIOS ELAFROPOULOS | 109 | -1 | . . . aacccctaccaactggtcggggtttgaaac |
| BBa_K5177024 | pTF_sfmA plasmid fragment cassette | DNA | Jia Run Dong | 351 | -1 | . . . tcaaaaccacgttgttttgaatttgaattc |
| BBa_K5177025 | Proposed pTF_fimA plasmid fragment cassette | DNA | Jia Run Dong | 351 | -1 | . . . cctacccaggttcagggacgtcatgaattc |
| BBa_K1026001 | dCas9 | Coding | Hongyi WU | 4113 | 2 | . . . ttgagtcagctaggaggtgactgagtcgac |
| BBa_K1137014 | tracRNA-CAS9 | Coding | Nicolas Koutsoubelis, Anne L�chner | 4522 | . . . aaaaaccccgcttcggcggggttttttttt | |
| BBa_K1559002 | rearranged CRISPR/Cas9 system without promoter | Coding | Xiuqi (Rex) Xia | 5080 | 6 | . . . aatttaaactttttattttaggaggcaaaa |
| BBa_K1559003 | CRISPR/Cas9 system with Anderson high-expression constitutive promoter | Generator | Xiuqi (Rex) Xia | 5127 | . . . aatttaaactttttattttaggaggcaaaa | |
| BBa_K1559004 | CRISPR/Cas9 system with Anderson medium-expression constitutive promoter | Generator | Xiuqi (Rex) Xia | 5127 | . . . aatttaaactttttattttaggaggcaaaa | |
| BBa_K1559005 | CRISPR/Cas9 system with Anderson low-expression constitutive promoter | Generator | Xiuqi (Rex) Xia | 5127 | . . . aatttaaactttttattttaggaggcaaaa | |
| BBa_K1559006 | CRISPR/Cas9 system with pBAD inducible promoter | Generator | Xiuqi (Rex) Xia | 5222 | . . . aatttaaactttttattttaggaggcaaaa | |
| BBa_K1559007 | CRISPR/Cas9 system with pLac inducible promoter | Generator | Xiuqi (Rex) Xia | 5147 | . . . aatttaaactttttattttaggaggcaaaa | |
| BBa_K1559008 | CRISPR/Cas9 system with pRha inducible promoter | Generator | Xiuqi (Rex) Xia | 5214 | . . . aatttaaactttttattttaggaggcaaaa | |
| BBa_K1774001 | Cas9 (optimized for expression in E. coli) | Coding | HKU iGEM 2015 | 4107 | 1 | . . . attgatctgagtcagctgggaggcgactaa |
| BBa_K1994015 | sgRNA with 5' golden gate adapter and COM protein binding site | RNA | Liam Carroll | 176 | . . . ggcacgtcatctgacgtgccttttttattt | |
| BBa_K1994016 | sgRNA with 5' golden gate adapter and MS2 coat protein binding site | RNA | Liam Carroll | 172 | . . . ggcacgtcatctgacgtgccttttttattt | |
| BBa_K1994019 | Multiple dCas9 binding site sequence | Regulatory | Liam Carroll | 239 | 2 | . . . atgattatcgttgttgctagccggcgtgga |
| BBa_K1982009 | Eukaryotic Cryptochrome 2 (CRY2) ( a blue light stimulated photoreceptor) | Coding | Zexu Li | 1848 | . . . actacaagtttgggaaaaaatggttgcaaa | |
| BBa_K1994044 | dCas9 Promoter | Regulatory | Egheosa Ogbomo | 48 | 3 | . . . tgaaatcatcaaactcattatggatttaat |
| BBa_K1982012 | VP64 transcription activitor | Protein_Domain | Zexu Li | 246 | . . . gactacaaggacgacgacgacaaataataa | |
| BBa_K2483002 | Lac regulated dCas9 with LacI constitutively expressed | Device | Bryan Nowack | 5674 | . . . attgatttgagtcagctaggaggtgactaa | |
| BBa_K2483004 | regulated dCas9 with sgRNAs and IAA enzymes fused to MS2 and PP7 | Composite | Bryan Nowack | 10136 | . . . ggcacgtcatctgacgtgccttttttattt | |
| BBa_K2558006 | gRNA targeting PhIF promotor | RNA | Tianze Huang | 96 | 3 | . . . caacttgaaaaagtggcaccgagtcggtgc |
| BBa_K2558007 | gRNA targeting lux pR and RiboJ | RNA | Tianze Huang | 96 | 2 | . . . caacttgaaaaagtggcaccgagtcggtgc |
| BBa_K3201000 | nCpf1 (RNA-guided DNA nickase) | Coding | Anastasios Galanis | 3921 | . . . tggctggcctacatccaggagctgcgcaac | |
| BBa_K3454000 | MCR-1_crRNA_A_Synthesis | Other | Mingxuan Chi | 63 | -1 | . . . acatctattgaccgcgaccgccaatcttac |
| BBa_K3454001 | MCR-1_crRNA_B_Synthesis | Other | Mingxuan Chi | 63 | -1 | . . . acatctactgacacttatggcacggtctat |
| BBa_K5490000 | 23-nt sequence binds CasRx to cleave WNV genome modifiable target 1. | RNA | IOANNIS VASILEIOS ELAFROPOULOS | 33 | -1 | . . . acaacagatgatttcgtgcaccagcttcca |
| BBa_K5490003 | 23-nt sequence binds CasRx to cleave WNV genome; modifiable target 3 | RNA | IOANNIS VASILEIOS ELAFROPOULOS | 43 | -1 | . . . gtacgtaatacccccaaagccgtacaagag |
British Columbia 2013
CRISPR used by UBCAbstract: The past decade has seen the emergence of robust bioprocessing strains engineered to synthesize discrete molecular products. The next-generation of strains could be “programmable,” with on demand generation of molecules within a bioreactor e.g. a yogurt fermentation capable of making any combination of flavouring, nutrients or pharmaceuticals. While merging all this potential into single hosts seems efficient, it would also bring added risk in the case of a process failure due to bacteriophage infection. Here, we not only rationally design widespread immunity to phage infection, but also hack this immunity system to yield programmable biosynthesis at the community level. We demonstrate this by building both broadly and specifically neutralizing CRISPR systems that were paired with biosynthetic capabilities for vanillin, caffeine and cinnamaldehyde production. Eventually, a fermentative process could exist that is vaccinated to phage infection but susceptible to targeted phage addition that results in a programmable probiotic – or ultrabiotic.
Chiba 2013
CRISPRi used by the Chiba teamAbstract: In nature, there exist a variety of magnetotactic bacteria. Recently, it was reported that non-magnetotactic cells such as yeast can be magnetized to some extent. We set the goal to transform E. coli into those that are attracted by magnets. By magnetizing E. coli, the cell harvesting process will be much simpler and more economical than the conventional processes such as centrifugation and filtration. To this end, we are conducting three itemized projects. (1) modification of iron transportation network to import as much Fe ions as possible in E. coli, (2) sequestering/ storing iron into human ferritin, and (3) converting cytosolic space from reducing to oxidizing in order to elevate Fe(II)/ Fe(III) ratio within. Because all such manipulations significantly impact the physiology of the host cell, we are establishing the BioBrick platform that enables the temporal knockdown of multiple genes using recently control technology such as CRISPRi.
Duke 2013
Abstract: Synthetic gene circuits have the potential to revolutionize gene therapies and bio-industrial methods by allowing predictable, customized control of gene expression. Bistable switches and oscillators, key building blocks of more complex gene networks, have been constructed using naturally occurring and well-characterized regulatory elements. In order to expand the versatility and variety of these circuits, we designed and constructed gene networks using artificial transcription factors (ATFs). The ATFs are of two classes: inhibitory TAL proteins and a catalytically inactive dCas9 protein with small guide RNA elements, each orthogonal to the yeast genome. Using mathematical modeling, we determined the parameters expected to create bistability and oscillation, using tandem binding site kinetics to achieve cooperativity. Based on these results, we assembled a library of plasmids containing ATFs, binding sites, regulatory elements, and fluorescent reporters. We then integrated these genes into the genome of Saccharomyces cerevisiae and are currently characterizing them using flow cytometry.
CRISPR References:
- Qi L.S, Larson M.H, Gilbert L.A, Doudna J.A, Weissman J.S, Arkin A.P, Lim W.A: Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152:1173-1183.
- DiCarlo J, Norville J, Mali P, Rios X, Aach J, Church M: Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Research 2013. 41(7), 4336-4343.
Freiburg 2013
Abstract: Our Team developed a universal toolkit, termed uniCAS, that enables customizable gene regulation in mammalian cells. Therefore, we engineered the recently discovered and highly promising CRISPR/Cas9 system. The regulation is based on the RNA-guided Cas9 protein, which allows targeting of specific DNA sequences. Our toolkit comprises not only a standardized Cas9 protein, but also different effector domains for efficient gene activation or repression. We further engineered a modular RNA plasmid for easy implementation of RNA guide sequences. As an additional feature, we established an innovative screening method for assessing the functionality of our uniCAS fusion proteins. Single genes and even whole genetic networks can be modified using our uniCAS toolkit. We think that our toolbox of standardized parts of the CRISPR/Cas9 system offers broad application in research fields such as tissue engineering, stem cell reprogramming and fundamental research.
Freiburg CRISPR References:
- Ishino, Y., et al. (1987). Nucleotide Sequence of the iap Gene in Escherichia coli. Journal of Bacteriology 169, 5429-5433.
- Barrangou, R., et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709-1712.
- Marraffini, L., and Sontheimer, E. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843-1845.
- Jansen, R., et al. (2002). Identification of genes that are associated with DNA repeats in prokaryotes. Molecular Microbiology 43, 1565-1575.
- Makarova, K., et al. (2011). Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9, 467-477.
- Jinek, M., et al. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816-821.
- Cong, L., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819-823.
- Mali, P., et al. (2013). RNA-guided human genome engineering via Cas9. Science 339, 823-826.
- Yamanaka, S., et al. (2012). Induced Pluripotent Stem Cells: Past, Present and Future. Cell Stem Cell 10, 678-684.
- Kaneshiro, K., et al. (2007). An integrated map of p53-binding sites and histone modification in the human ENCONDE regions. Genomics, 177-188.
MIT 2013
Abstract: Coordinating behavior across cell populations to form synthetic tissues requires spacial communication between individual cells. While there has been some success engineering single signals, sending multiple signaling elements spanning spatial scales for multicellular coordination remains a significant hurdle. Here, we describe a method for mammalian cell-cell communication utilizing engineered exosomes containing miRNA or protein signals. First, we demonstrate selectively packaging signaling miRNAs (miR-451 and miR-503) and synthetic fusion proteins (GFP, Cas9, and Cre recombinase each individually fused to the oligomerizing membrane targeting domain Acyl-TyA) into exosomes within cells engineered with sender genetic circuits. Next, we demonstrate that these miRNA and protein signals can modulate gene expression within cells engineered with receiver genetic circuits. Finally, we present preliminary cell-cell signaling results on populations of cocultured sender and receiver cells. Our method may enable multiplexed communication among populations of various cell types and the creation of sophisticated synthetic tissues.
MIT CRISPR references:
- Bacchus, William et al. Synthetic two-way communication between mammalian cells. Nat. Biotechnol. 30, 991–996 (2012)
- Lancaster, Madeline et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013)
- Barrangou, Rodolphe et al. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 314, 1709-1712 (2007)
- Shen, B et al. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. (2011)
NJU NJUT China 2013
Abstract: Most bacteria and archaea can resist invading DNA and/or RNA elements via the clusters of regularly interspaced short palindromic repeats (CRISPRs).It is believed that the integrated CRISPR sequences have the ability to form a genetic memory which prevents the host from being infected.The memory exist as a DNA library in genome, artificially modified to set its target. The CRISPRs and Cas (CRISPR-associated) interact and form this prokaryotic adaptive immune system. Cas9, as a core of CRISPR system, can play a role of targeted-attacking gene "missiles". Therefore, we build a sort of plasmids, loading CRISPR system, to realize the "killing" of harmful genes and/or organisms.
CRISPR and Cas9 references:
- Zhang J, Rouillon C, Kerou M, et al. Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity[J]. Molecular cell, 2012, 45(3): 303-313.
- Brouns S J J, Jore M M, Lundgren M, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes[J]. Science, 2008, 321(5891): 960-964.
- Díez-Villaseñor C, Almendros C, García-Martínez J, et al. Diversity of CRISPR loci in Escherichia coli[J]. Microbiology, 2010, 156(5): 1351-1361.
- Marraffini L A, Sontheimer E J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA[J]. science, 2008, 322(5909): 1843-1845.
- Shen B, Zhang J, Wu H, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting[J]. Cell research, 2013.
- Westra E R, Swarts D C, Staals R H J, et al. The CRISPRs, they are a-Changin': how prokaryotes generate adaptive immunity[J]. Annual review of genetics, 2012, 46: 311-339.
- Cong L, Ran F A, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems[J]. Science, 2013, 339(6121): 819-823.
- Karginov F V, Hannon G J. The CRISPR system: small RNA-guided defense in bacteria and archaea[J]. Molecular cell, 2010, 37(1): 7-19.
- Mali P, Yang L, Esvelt K M, et al. RNA-guided human genome engineering via Cas9[J]. Science, 2013, 339(6121): 823-826.
- Barrangou R, Horvath P. CRISPR: new horizons in phage resistance and strain identification[J]. Annual review of food science and technology, 2012, 3: 143-162.
- Hale C R, Majumdar S, Elmore J, et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs[J]. Molecular cell, 2012, 45(3): 292-302.
- Semenova E, Jore M M, Datsenko K A, et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence[J]. Proceedings of the National Academy of Sciences, 2011, 108(25): 10098-10103.
Paris Bettencourt 2013
Abstract: We are testing new weapons for the global war against Mycobacterium tuberculosis (MTb), a pathogen that infects nearly 2 billion people. Our 4 synergistic projects aim to help in the prevention, diagnosis, and treatment of tuberculosis. 1) We are reproducing an essential MTb metabolic pathway in E. coli, where it can be easily and safely targeted in a drug screen. 2) We are building a phage-based biosensor to allow the rapid diagnosis specifically drug-resistant MTb strains. 3) We are constructing a mycobacteriophage to detect and counterselect drug-resistant Mtb in the environment. 4) We are programming E. coli to follow MTb into human macrophages and saturate it with bacteriolytic enzymes. We want to vanquish tuberculosis and build a TB-free world.
Penn State 2013
Penn State plan Cas9 project information
Abstract: Plants as Plants: Natural Factories provides a green approach to the manufacturing of valuable chemicals and materials. Through synthetic biology, we are able to control the expression of genes that regulate the production of desired secondary metabolites. Via the manipulation of established metabolic pathways, we hope to produce vanillin and butanol. The prospect of being able to synthetically produce a biofuel provides vast possibilities for the scope of synthetic biology and green energy. Additionally through the manipulation of the cellulose synthase genes, we hope to increase the biomass of plants by a hybrid plant cell wall. As shown through these projects, the use of plants provides various green energy possibilities. However, due to the limited use of plants within synthetic biology there are various regulation issues. Thus we have additionally worked on characterizing a range of plant promoters as well as introducing the Cas9 crisper system into plants.
SJTU BioX Shanghai 2013
SJTU-BioX-Shanghai CRISPR page
Abstract: Few researches have been done to regulate gene expression levels in genomic scale so far. This year we aim to combine two systems together in order to provide a universal and convenient tool which can be used to regulate different genomic genes simultaneously and independently in a quantitative way. Our project involves the newly developed gene regulating tool CRISPRi and three light-controlled expression systems induced by red, green, and blue light respectively. Simply by changing the regulating parts in CRISPRi system towards mRFP, luciferase, and three enzymes, we hope to prove our system can be used qualitatively, quantitatively and practically step by step. We have also designed a box and written a software as our experiment measurements. Simply by typing in several parameters, different gene expression levels can be controlled. This system can also be improved to predict the maximized producing efficiency after some simple tests in future.
SJTU-BioX-Shanghai CRISPR references:
- QI, LEI S., LARSON, MATTHEW H., GILBERT, LUKE A., DOUDNA, JENNIFER A., WEISSMAN, JONATHAN S., ARKIN, ADAM P. & LIM, WENDELL A. 2013. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell, 152, 1173-1183
- LEVSKAYA, A., CHEVALIER, A. A., TABOR, J. J., SIMPSON, Z. B., LAVERY, L. A., LEVY, M., DAVIDSON, E. A., SCOURAS, A., ELLINGTON, A. D., MARCOTTE, E. M. & VOIGT, C. A. 2005. Synthetic biology: engineering Escherichia coli to see light. Nature, 438, 441-2.
- FU, Y., FODEN, J. A., KHAYTER, C., MAEDER, M. L., REYON, D., JOUNG, J. K. & SANDER, J. D. 2013. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nature Biotechnology, 31, 822-826.
- CARROLL, D. 2013. Staying on target with CRISPR-Cas. Nature Biotechnology, 31, 807-809.
Stanford Brown 2013
Abstract: Communication is a dynamic requirement for life as we know it. We are using cellular and molecular messaging of different magnitudes to improve the broadcasting and reception of information. Starting on the atomic level, our BioWires project has created silver-incorporating DNA to act as nanowires, which could improve the cost and effectiveness of electronic products. Our CRISPR project is creating a system for DNA messages and resistances to be passed from cell to cell, in effect, creating transmissible probiotics and changing the way that cells communicate. We are also building a chromogenic biosensor to detect sucrose secretion that will be launched on a satellite (EuCROPIS) into low-Earth orbit. Finally, our De-Extinction project involves decoding messages from the past to better understand early life on Earth.
Stanford-Brown CRISPR references:
- Bikard, D., Jiang, W., Samai, P., Hochschild, A., Zhang, F., & Marraffini, L. A. (2013). Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Research.
- Deltcheva, E., Chylinski, K., Sharma, C. M., Gonzales, K., Chao, Y., Pirzada, Z. A., ... & Charpentier, E. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 471(7340), 602-607.
- Jiang, W., Bikard, D., Cox, D., Zhang, F., & Marraffini, L. A. (2013). RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature biotechnology, 31(3), 233-239.
- Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816-821.
- Liu, H., & Naismith, J. H. (2008). An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC biotechnology, 8(1), 91.
- Mali, P., Yang, L., Esvelt, K. M., Aach, J., Guell, M., DiCarlo, J. E., ... & Church, G. M. (2013). RNA-guided human genome engineering via Cas9. Science, 339(6121), 823-826.
- Qi, L. S., Larson, M. H., Gilbert, L. A., Doudna, J. A., Weissman, J. S., Arkin, A. P., & Lim, W. A. (2013). Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell, 152(5), 1173-1183.
- Quan, J., & Tian, J. (2009). Circular polymerase extension cloning of complex gene libraries and pathways. PloS one, 4(7), e6441.
- Wang, H., Yang, H., Shivalila, C. S., Dawlaty, M. M., Cheng, A. W., Zhang, F., & Jaenisch, R. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell.
UCSF 2013
Abstract: In microbial communities, bacterial populations are commonly controlled using indiscriminate, broad range antibiotics. There are few ways to target specific strains effectively without disrupting the entire microbiome and local environment. The goal of our project is to take advantage of a natural horizontal gene transfer mechanism in bacteria to precisely affect gene expression in selected strains. We combine bacterial conjugation with CRISPRi, an RNAi-like repression system developed from bacteria, to regulate gene expression in targeted strains within a complex microbial community. One possible application is to selectively repress pathogenic genes in a microbiome, leaving the community makeup unaffected. In addition, we use CRISPRi to lay the groundwork for transferring large circuits that enable complex functionality and decision-making in cells.
WHU China 2013
Abstract: Cas9 is an RNA-guided dsDNA nuclease utilized by bacteria immune system. The genetically engineered Cas9 has recently been shown to have the ability to repress or activate desired gene expression. In practical research and industrial application, we usually face the problem to express a gene at different levels, not only “on” or “off ”, so a more flexible regulation method is needed. To achieve multi-stage regulation of target genes, we further develop several dCas9 devices in which dCas9 alone or fused with omega subunit of RNAP is directed by various guide RNAs to different regions of designed double promoters. Therefore, promoters with disparate strength can be either activated or repressed respectively and multi-stage gene expression can be achieved. Also, based on such novel technology platform, we are developing diverse applications such as a guide RNA-mediated oscillator.
WHU-China CRISPR references:
- Qi, L.S., et al., Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell, 2013. 152(5): p. 1173-83.
- Jinek, M., et al., A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 2012. 337(6096): p. 816-21.
- Bikard, D., et al., Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res, 2013. 41(15): p. 7429-7437.
- Heidrich, N. and J. Vogel, CRISPRs extending their reach: prokaryotic RNAi protein Cas9 recruited for gene regulation. EMBO J, 2013. 32(13): p. 1802-4.
- Li, M., et al., A strategy of gene overexpression based on tandem repetitive promoters in Escherichia coli. Microb Cell Fact, 2012. 11: p. 19.