Difference between revisions of "Part:BBa K1028001"
(→Usage and Experimental Data) |
(→Scanning Electron Microscopy Imaging (SEM)) |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<br> | <br> | ||
=== '''Introduction''' === | === '''Introduction''' === | ||
| − | http://i276.photobucket.com/albums/kk29/JosephBeetBeet/ | + | This BioBrick encodes for a sequence that displays the silica specific Si4 binding peptide, using Ice Nucleation Protein (INP), on the exterior surface of E. coli cells resulting in binding with high affinity via the INP.Si4 complex. |
| − | < | + | |
| − | + | INP is known to display sequences downstream of the C-terminus in the extra-cellular space via acting as a trans-membrane protein. | |
| + | |||
| + | The silica binding peptide is part [https://parts.igem.org/Part:BBa_K1028000 BBa_K1028000] and the INP is part [https://parts.igem.org/Part:BBa_K811005 BBa_K811005] | ||
| + | |||
| + | <html> | ||
| + | <img src="http://i276.photobucket.com/albums/kk29/JosephBeetBeet/INPSi4Complex.png" style="width:400px;margin-left:160px" ></a> | ||
| + | |||
| + | <p style="text-align:left"><b>Fig.1</b> Illustrated schematic of the INP.Si4 complex showing how it is formed and displayed on the outside of the cell. | ||
| + | </p></html> | ||
=== '''Usage and Experimental Data''' === | === '''Usage and Experimental Data''' === | ||
| + | ====Growth Curve ==== | ||
<html> | <html> | ||
<img src="http://i276.photobucket.com/albums/kk29/JosephBeetBeet/INPSi4Growthcurve.png" style="width:400px;margin-right:160px" ></a> | <img src="http://i276.photobucket.com/albums/kk29/JosephBeetBeet/INPSi4Growthcurve.png" style="width:400px;margin-right:160px" ></a> | ||
| − | <p style="text-align:center"><b>Fig.1:</b> Monitoring the growth rate of cells containing Si4 Binding Peptide attached to the C-terminus of INP, labelled here as Device 2, within the PSB1C3 standard iGEM submission plasmid. Although the graph shows a log and a lag phase for the cells containing the insert plasmid, the absorbance reached in log phase is very low compared to the control cells. | + | <p style="text-align:center"><b>Fig.1:</b> Monitoring the growth rate of cells containing Si4 Binding Peptide attached to the C-terminus of INP, labelled here as Device 2, within the PSB1C3 standard iGEM submission plasmid. Although the graph shows a log and a lag phase for the cells containing the insert plasmid, the absorbance reached in log phase is very low compared to the control cells in fact showing the poorest growth of any of the Leeds 2013 submitted parts. |
</p></html> | </p></html> | ||
| + | ====Fluorescent Activated Cell Sorting (FACS) ==== | ||
| + | <html> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/thumb/b/be/Leeds_FACS_Overall_Information_Pic.png/800px-Leeds_FACS_Overall_Information_Pic.png" style="width:800px;margin-right:160px" ></a> | ||
| + | |||
| + | <p style="text-align:center"><b>Fig.2:</b> INP.Si4, labelled here as Device 2 which also contains GFP coding region under the control of the CpxR promoter, characterized using FACS. This data shows successful binding of a small portion of our expression cells to the silica beads used, this result is discussed in more depth below. | ||
| + | </p></html> | ||
| + | |||
| + | Device 2 contains our INP+Si4 construct, along with the Cpx and GFP genes. It was characterized using a flow cytometry method called Fluorescence-activated cell sorting (FACS) in solution with and without beads. The FACS images contain Side scattering and Forward scattering information, relating to relative size and density of the cells. | ||
| + | |||
| + | In the assay containing Device 2 with beads, there was an increase in the percentage of Population 3 (coloured in blue), from 0.1 to 2.2. This is suggestive of representing our Device 2 cells bound to silica beads. In contrast, the assay, which contained only Device 2 cells, had a very insignificant percentage of Population 3 (0.1%). | ||
| + | |||
| + | This suggests that our Device may successfully be binding to the silica beads utilizing the INP+Si4 construct, however not in all cases. This could either be due to not having enough time for the Device to binding to the silica, or that our Si4 binding domain is not as effective as it should be. | ||
| + | |||
| + | ====Scanning Electron Microscopy Imaging (SEM)==== | ||
| + | |||
| + | |||
| + | <html> | ||
| + | <img src="http://i276.photobucket.com/albums/kk29/JosephBeetBeet/SEMImage2FromOleg-1mMTeOS.png" style="width:800px;margin-right:160px" ></a> | ||
| + | |||
| + | <p style="text-align:left"><b>Fig.3</b> SEM image showing expression cells containing Device 2 in solution with 1mM TeOS. | ||
| + | </p></html> | ||
| + | |||
| + | The image above contains images that were produced by the Scanning Electron Microscope (SEM) at the University of Leeds. Device 2 was incubated in solution of varying concentrations of an alkylsilane compound, which was solubilized using the sol-gel method from Tetraethoxysilane (TEOS). Samples were incubated at room temperature, as well as some were done at 45ºC. For the control samples, cells without the Device 2 construct gene were used, and did not react with the alkylsilane compound. | ||
| + | |||
| + | The alkylsilane compound forming distinct spheres can be witnessed in one of the images. EDX chemical composition data is also included with each image, which allows assessment of the elements that are present in each sample. The high carbon output is partially due to the carbon adhesive used to isolate the samples for SEM. The alkylsilane compound would envelop the cells either fully or partially and this can be gathered from the images shown. The images of control samples exemplify the alkylsilane compound not extensively bound to the cells. | ||
| + | <br> | ||
| + | <br> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Latest revision as of 18:21, 4 October 2013
INP.Si4 Silica Binding Complex
Introduction
This BioBrick encodes for a sequence that displays the silica specific Si4 binding peptide, using Ice Nucleation Protein (INP), on the exterior surface of E. coli cells resulting in binding with high affinity via the INP.Si4 complex.
INP is known to display sequences downstream of the C-terminus in the extra-cellular space via acting as a trans-membrane protein.
The silica binding peptide is part BBa_K1028000 and the INP is part BBa_K811005
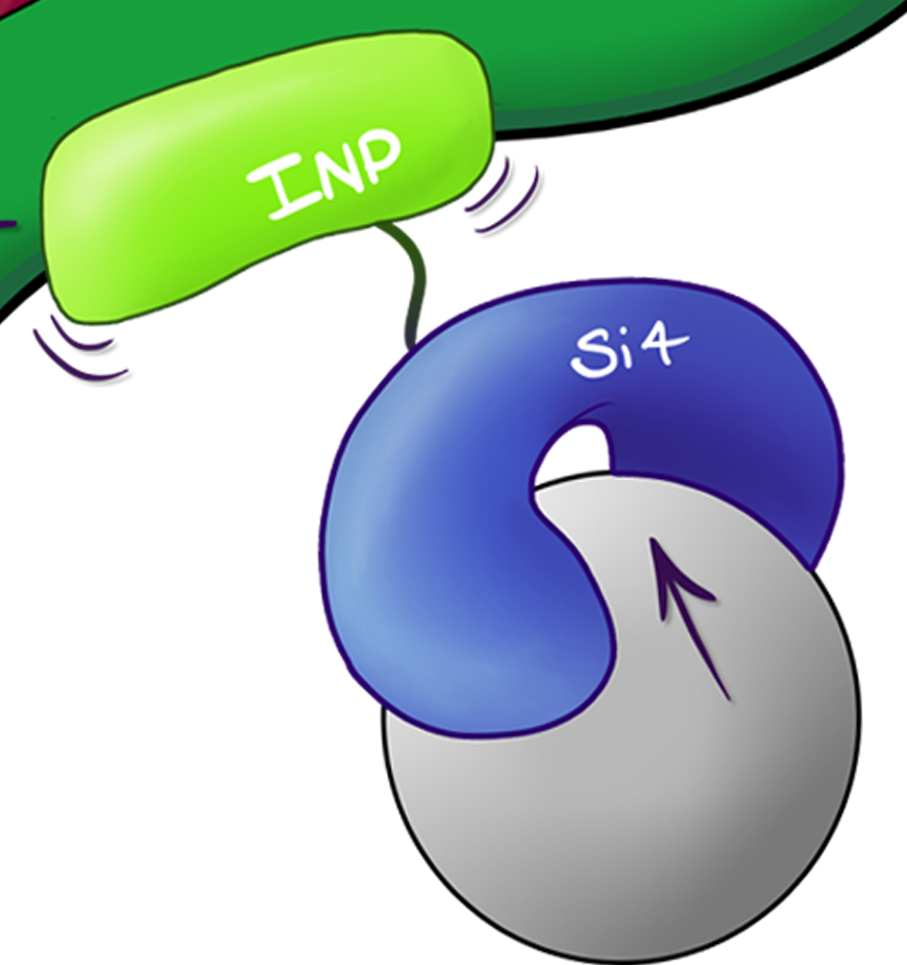
Fig.1 Illustrated schematic of the INP.Si4 complex showing how it is formed and displayed on the outside of the cell.
Usage and Experimental Data
Growth Curve
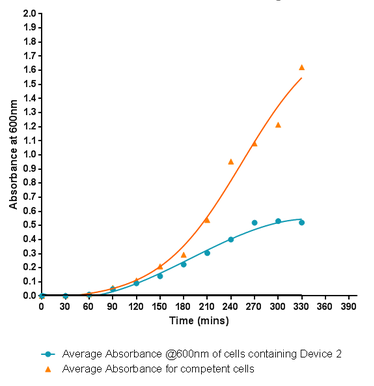
Fig.1: Monitoring the growth rate of cells containing Si4 Binding Peptide attached to the C-terminus of INP, labelled here as Device 2, within the PSB1C3 standard iGEM submission plasmid. Although the graph shows a log and a lag phase for the cells containing the insert plasmid, the absorbance reached in log phase is very low compared to the control cells in fact showing the poorest growth of any of the Leeds 2013 submitted parts.
Fluorescent Activated Cell Sorting (FACS)

Fig.2: INP.Si4, labelled here as Device 2 which also contains GFP coding region under the control of the CpxR promoter, characterized using FACS. This data shows successful binding of a small portion of our expression cells to the silica beads used, this result is discussed in more depth below.
Device 2 contains our INP+Si4 construct, along with the Cpx and GFP genes. It was characterized using a flow cytometry method called Fluorescence-activated cell sorting (FACS) in solution with and without beads. The FACS images contain Side scattering and Forward scattering information, relating to relative size and density of the cells.
In the assay containing Device 2 with beads, there was an increase in the percentage of Population 3 (coloured in blue), from 0.1 to 2.2. This is suggestive of representing our Device 2 cells bound to silica beads. In contrast, the assay, which contained only Device 2 cells, had a very insignificant percentage of Population 3 (0.1%).
This suggests that our Device may successfully be binding to the silica beads utilizing the INP+Si4 construct, however not in all cases. This could either be due to not having enough time for the Device to binding to the silica, or that our Si4 binding domain is not as effective as it should be.
Scanning Electron Microscopy Imaging (SEM)

Fig.3 SEM image showing expression cells containing Device 2 in solution with 1mM TeOS.
The image above contains images that were produced by the Scanning Electron Microscope (SEM) at the University of Leeds. Device 2 was incubated in solution of varying concentrations of an alkylsilane compound, which was solubilized using the sol-gel method from Tetraethoxysilane (TEOS). Samples were incubated at room temperature, as well as some were done at 45ºC. For the control samples, cells without the Device 2 construct gene were used, and did not react with the alkylsilane compound.
The alkylsilane compound forming distinct spheres can be witnessed in one of the images. EDX chemical composition data is also included with each image, which allows assessment of the elements that are present in each sample. The high carbon output is partially due to the carbon adhesive used to isolate the samples for SEM. The alkylsilane compound would envelop the cells either fully or partially and this can be gathered from the images shown. The images of control samples exemplify the alkylsilane compound not extensively bound to the cells.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 409
- 1000COMPATIBLE WITH RFC[1000]
