Difference between revisions of "Part:BBa K818000:Experience"
| (22 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
===User Reviews=== | ===User Reviews=== | ||
| − | <!-- DON'T DELETE --><partinfo> | + | <!-- DON'T DELETE --><partinfo>BBa_E0040 StartReviews</partinfo> |
<!-- Template for a user review | <!-- Template for a user review | ||
{|width='80%' style='border:1px solid gray' | {|width='80%' style='border:1px solid gray' | ||
| Line 13: | Line 13: | ||
|width='10%'| | |width='10%'| | ||
<partinfo>BBa_K818000 AddReview number</partinfo> | <partinfo>BBa_K818000 AddReview number</partinfo> | ||
| − | <I>Newcastle University | + | <I>UsernEnter the review inofrmation here.ame</I> |
| + | |width='60%' valign='top'| | ||
| + | |||
| + | |}; | ||
| + | <!-- End of the user review template --> | ||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_K818000 1</partinfo> | ||
| + | <I>Newcastle University iGEM 2013</I> | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
This BioBrick was designed to be used as an integration backbone for the ‘’B. subtilis’’ by integrating at the ‘’sacA’’ region of the endogenous chromosome via double crossover. | This BioBrick was designed to be used as an integration backbone for the ‘’B. subtilis’’ by integrating at the ‘’sacA’’ region of the endogenous chromosome via double crossover. | ||
| Line 19: | Line 29: | ||
We transformed this backbone into ‘’B. subtilis’’ but as can be seen in Figure 1, no colonies were found on the ‘’B. subtilis’’ str. 168 + pSac-Cm derived integration plasmid, however the positive control ‘’B. subtilis’’ str.168 + pGFPrrnB (integrates at amyE) did work, which suggested that this backbone was not integrated. | We transformed this backbone into ‘’B. subtilis’’ but as can be seen in Figure 1, no colonies were found on the ‘’B. subtilis’’ str. 168 + pSac-Cm derived integration plasmid, however the positive control ‘’B. subtilis’’ str.168 + pGFPrrnB (integrates at amyE) did work, which suggested that this backbone was not integrated. | ||
| + | |||
| + | <html> | ||
| + | <style type="text/css"> | ||
| + | #body img{ | ||
| + | margin-bottom:3px; | ||
| + | } | ||
| + | table{ | ||
| + | border:2px solid black; | ||
| + | margin-left:50px; | ||
| + | margin-bottom: 10px; | ||
| + | width: 650px; | ||
| + | } | ||
| + | .italic{ | ||
| + | font-style: italic; | ||
| + | } | ||
| + | table.borderless { | ||
| + | border:0px solid white; | ||
| + | } | ||
| + | |||
| + | </style> | ||
| + | <body> | ||
| + | <br> | ||
| + | <table border="0"> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/1/10/BareCillus_Rrnb_1.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">Plate 1: B. Subtilis str. 168 transformed with pGFPrrnB.</div></td> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/8/89/BareCillus_H20_1.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">Plate 2: B.subtilis str. 168 transformed with H20(negative control).</div></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/6/67/BareCillus_Gro_1.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">Plate 3: B. subtilis str. 168 with pSac + Cm derived plasmid. | ||
| + | </div></td> | ||
| + | <td><div class="italic">Figure 1. Plates of B. Subtilis str. 168 transformed with the pSac+Cm derived plasmid, pGFPrrnB (positive control) and water (negative control).</div></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </body> | ||
| + | </html> | ||
We repeated the transformations using higher concentration of plasmids 5ug, 10ug, and 15ug and plated them onto the LB + 5ug/ml Chloramphenicol plates. The results Figure 2, shows that there were colonies growing on the 10ug and 15ug plates. | We repeated the transformations using higher concentration of plasmids 5ug, 10ug, and 15ug and plated them onto the LB + 5ug/ml Chloramphenicol plates. The results Figure 2, shows that there were colonies growing on the 10ug and 15ug plates. | ||
| + | <html> | ||
| + | <style type="text/css"> | ||
| + | #body img{ | ||
| + | margin-bottom:3px; | ||
| + | } | ||
| + | table{ | ||
| + | border:2px solid black; | ||
| + | margin-left:50px; | ||
| + | margin-bottom: 10px; | ||
| + | width: 650px; | ||
| + | } | ||
| + | .italic{ | ||
| + | font-style: italic; | ||
| + | } | ||
| + | |||
| + | </style> | ||
| + | <body> | ||
| + | <table border="0"> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/6/69/BareCillus_Gro5.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">B. subtilis str. 168 with 5ug pSac + Cm derived plasmid.</div></td> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/7/72/BareCillus_Gro10.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">B. subtilis str. 168 with 10ug pSac + Cm derived plasmid.</div></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/7/74/BareCillus_Gro15.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">B. subtilis str. 168 with 15ug pSac + Cm derived plasmid.</div></td> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/8/89/BareCillus_H20_1.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">B.subtilis str. 168 transformed with H20(negative control)</div></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><img src="https://static.igem.org/mediawiki/parts/6/63/Rrnb_2.jpg" alt="Pulpit rock" width="304" height="228"><div class="italic">B. Subtilis str. 168 transformed with pGFPrrnB.</div></td> | ||
| + | <td><div class="italic">Figure 1. Plates of B. Subtilis str. 168 transformed with 5,10 and 15ug pSac+Cm derived plasmid, pGFPrrnB (positive control) and water (negative control).</div></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </body> | ||
| + | </html> | ||
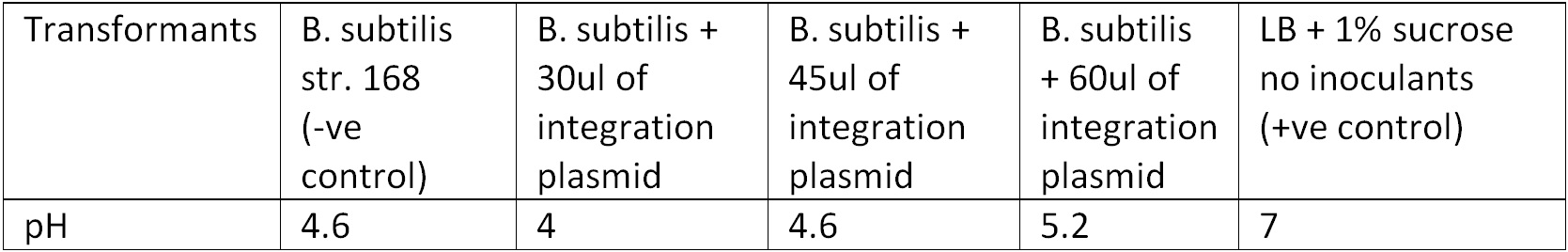
To test for integration, we used the Phenol red Sucrose test; the media where the transformants from the 10ug and 15ug plates were inoculated showed the same results as the control (‘’B. subtilis’’ + pGFPrrnB) with pH ranging between 4.6 – 5.2 suggesting that they could utilized sucrose as their carbon source and produced the acid by products, Figure 3. | To test for integration, we used the Phenol red Sucrose test; the media where the transformants from the 10ug and 15ug plates were inoculated showed the same results as the control (‘’B. subtilis’’ + pGFPrrnB) with pH ranging between 4.6 – 5.2 suggesting that they could utilized sucrose as their carbon source and produced the acid by products, Figure 3. | ||
| − | + | ||
| − | + | [[File:BareCillus_Gro_pH.jpg|900px]] | |
| − | + | ||
| − | + | ||
Figure 3. Display the pH of each samples following the Phenol Red Sucrose test on overnight grown culture in LB + 1% Sucrose media. | Figure 3. Display the pH of each samples following the Phenol Red Sucrose test on overnight grown culture in LB + 1% Sucrose media. | ||
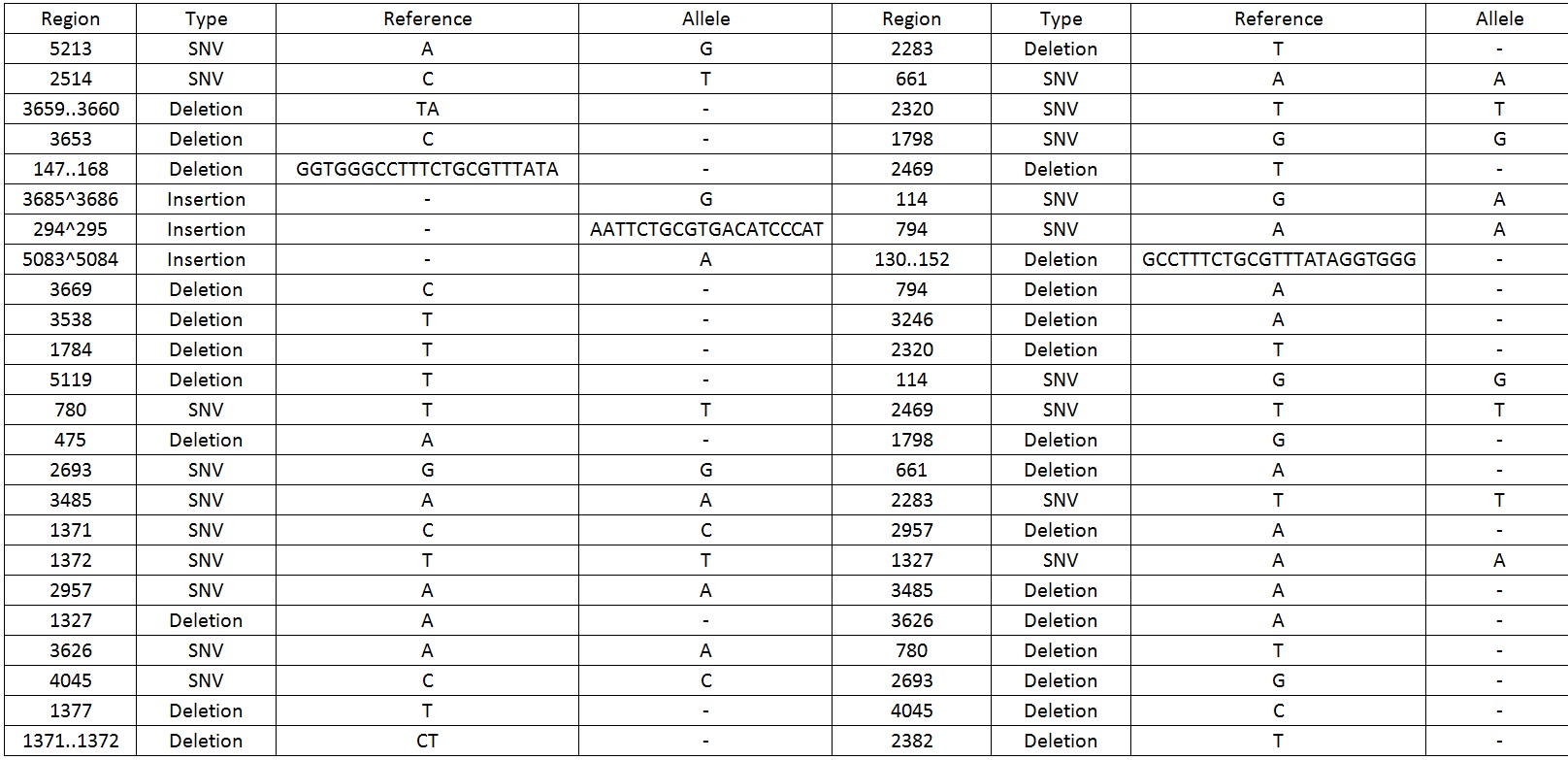
| − | We then sequenced the backbone and found out that there were 49 mutations which include SNV, deletions and insertions | + | We then sequenced the backbone and found out that there were 49 mutations which include SNV, deletions and insertions.Table 1 display the mutations found on the sequence. |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| + | [[File:BareCillus_Gro_mutations.jpg|700px]] | ||
Table 1. Shows all the mutations found in the BBa_K818000 backbone. | Table 1. Shows all the mutations found in the BBa_K818000 backbone. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
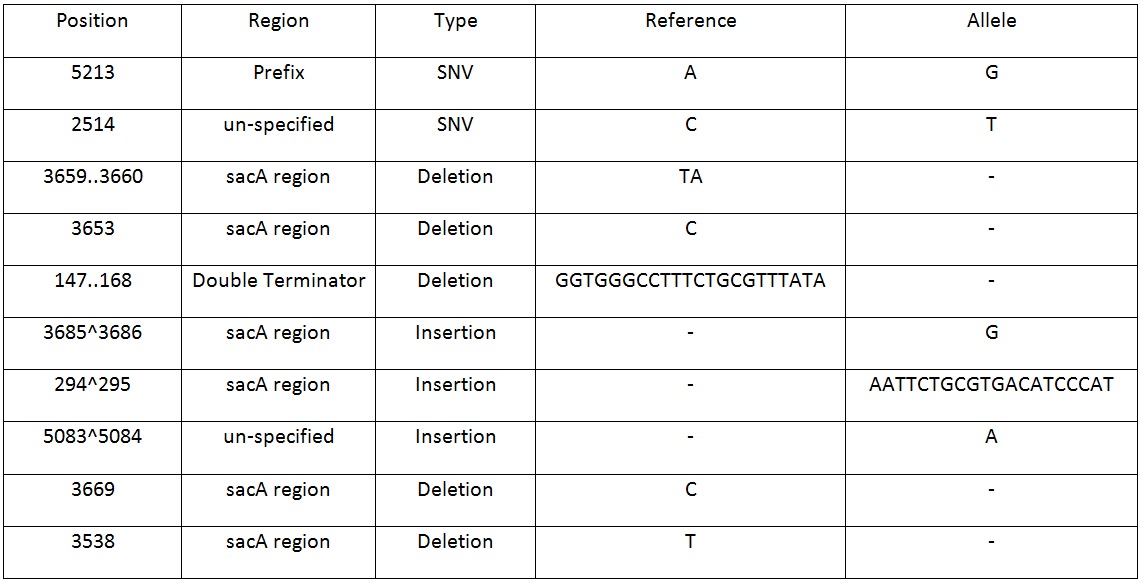
Table 2. Shows list of mutations found in the BBa_K818000 backbone, including the position, region and type of mutations after analysing the initial sequencing and the sequencing of highly mutated regions. | Table 2. Shows list of mutations found in the BBa_K818000 backbone, including the position, region and type of mutations after analysing the initial sequencing and the sequencing of highly mutated regions. | ||
| + | [[File:BareCillus Gro sumseq.jpg|700px]] | ||
| + | Table 2. Shows list of mutations found in the BBa_K818000 backbone, including the position, region and type of mutations after analysing the initial sequencing and the sequencing of highly mutated regions. | ||
| + | |||
| + | [[File:Gro analysis high thgrougput.png|700px]] | ||
| + | |||
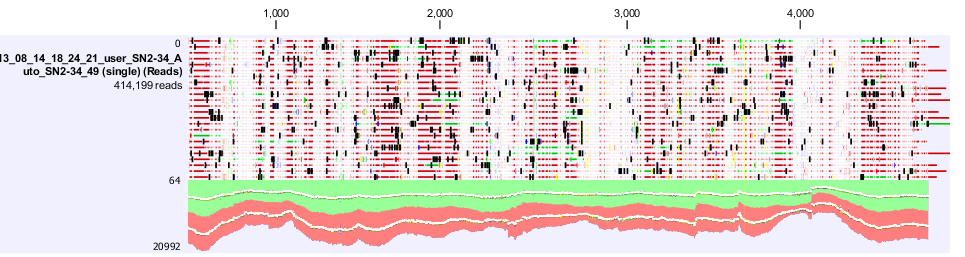
| + | Figure 4. shows a screen shot of the pSac-Cm derived integration plasmid sequence analysis that we performed. It shows the amount of coverage, where the mutations are. | ||
The results from both sequencing run proved to show similar mutations were found on this backbone, most of the mutations occurred in the sacA integration regions. These results explained the reason why this pSac-Cm derived integration backbone for ‘’B.subtilis’’ were not working. | The results from both sequencing run proved to show similar mutations were found on this backbone, most of the mutations occurred in the sacA integration regions. These results explained the reason why this pSac-Cm derived integration backbone for ‘’B.subtilis’’ were not working. | ||
To get round this problem, we align the sequence of this BioBrick to the Integration vector pSac-Cm sequence from the (Middleton, R., Hofmeister, A. New shuttle vectors for ectopic insertion of genes into Bacillus subtilis. Plasmid Volume 51, Issue 3, May 2004, Pages 238–245). The results showed that the sequence that the Groningen team 2012 put up on the registry was correct. This suggests that the plasmid that they have submitted and the sequence they provided did not match. By using the correct sequence to generate this integration plasmid we will be able to make this part functional not just in ‘’E. coli’’ but also ‘’B. subtilis. | To get round this problem, we align the sequence of this BioBrick to the Integration vector pSac-Cm sequence from the (Middleton, R., Hofmeister, A. New shuttle vectors for ectopic insertion of genes into Bacillus subtilis. Plasmid Volume 51, Issue 3, May 2004, Pages 238–245). The results showed that the sequence that the Groningen team 2012 put up on the registry was correct. This suggests that the plasmid that they have submitted and the sequence they provided did not match. By using the correct sequence to generate this integration plasmid we will be able to make this part functional not just in ‘’E. coli’’ but also ‘’B. subtilis. | ||
| + | |||
|}; | |}; | ||
| − | |||
| − | |||
Latest revision as of 17:45, 4 October 2013
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K818000
This backbone plasmid was used as primary backbone for all constructs in team Groningen 2012 project: the Food Warden. For further info: [http://2012.igem.org/Team:Groningen/OurBiobrick iGEM Groningen 2012 biobrick page]
User Reviews
UNIQ984400a66af36de4-partinfo-00000000-QINU