Difference between revisions of "Part:BBa I13453"
(→Usage and Biology: - copied 3 bullet points from OWW article) |
|||
| (13 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_I13453 short</partinfo> | <partinfo>BBa_I13453 short</partinfo> | ||
| Line 8: | Line 7: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | Has been used as a second promoter in a system containing I0500 (PBad+AraC). In this system, it showed behavior qualitatively indistinguishable from the I0500 copy of PBad. Has not been tested independent of AraC. A second part, I13458, should allow decoupling of PBad and AraC. | + | Has been used as a second promoter in a system containing <partinfo>I0500</partinfo> (PBad+AraC). In this system, it showed behavior qualitatively indistinguishable from the <partinfo>I0500</partinfo> copy of PBad. Has not been tested independent of AraC. A second part, <partinfo>I13458</partinfo>, should allow decoupling of PBad and AraC. |
| − | + | ||
| − | + | See this OpenWetWare article on [http://openwetware.org/wiki/Titratable_control_of_pBAD_and_lac_promoters_in_individual_E._coli_cells#pBAD_promotersOpenWetWare pBAD and lac promoters] for additional usage and biology information | |
| − | + | ||
| − | + | ==2024 Squirrel-CHN supplementary applications== | |
| + | ===Usage and Biology=== | ||
| + | First, we induced the expression of red fluorescent protein using the arabinose operon to verify its functionality in E. coli DH5α. | ||
| + | Then, our team used the arabinose operon to induce the expression of the bacteriolytic proteins T4 holin and T4 lysozyme, with BBa_B0015 as the terminator. We recombined the fragments into the plasmid pET23b and transformed the constructed plasmid into E. coli DH5α. | ||
| + | <html> | ||
| + | <div style="display:flex; flex-direction: column; align-items: center;"> | ||
| + | <img src="https://static.igem.wiki/teams/5083/16.png" style="width: 500px;margin: 0 auto" /> | ||
| + | <p style="font-size: 98%; line-height: 1.4em;">Fig 1.genetic circuit diagram</p > | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | ===Potential application directions=== | ||
| + | Firstly, we performed sequence amplification of the arabinose operon, bacteriolytic protein T4 holin, and T4 lysozyme. | ||
| + | <html> | ||
| + | <div style="display:flex; flex-direction: column; align-items: center;"> | ||
| + | <img src="https://static.igem.wiki/teams/5083/6.png" style="width: 500px;margin: 0 auto" /> | ||
| + | <p style="font-size: 98%; line-height: 1.4em;">Fig 2.Agarose gel electrophoresis to verify the sequence amplification</p > | ||
| + | </div> | ||
| + | </html> | ||
| + | Secondly, we validated the functionality of the arabinose operon in E. coli DH5α. The results showed that as the concentration of arabinose increased, the Fluorescence/OD600 ratio increased, demonstrating that the arabinose operon can effectively induce the expression of the red fluorescent protein. | ||
| + | |||
| + | Finally, we introduced the suicide system into E. coli DH5α. The results indicated that under induction with 0.5 mM/L arabinose, the OD600 of the bacterial culture containing the arabinose operon suicide system was significantly lower than that of the control, proving the effectiveness of the suicide system. | ||
| + | <html> | ||
| + | <div style="display:flex; flex-direction: column; align-items: center;"> | ||
| + | <img src="https://static.igem.wiki/teams/5083/17.png" style="width: 500px;margin: 0 auto" /> | ||
| + | <p style="font-size: 98%; line-height: 1.4em;">Fig 3.Verification of the Arabinose Operon Suicide System(A) Fluorescence/OD600 ratio of red fluorescent protein expression induced by different concentrations of arabinose operon(B) Growth of E. coli DH5α under 0.5 mM/L arabinose</p > | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_I13453 SequenceAndFeatures</partinfo> | <partinfo>BBa_I13453 SequenceAndFeatures</partinfo> | ||
| + | ===Characterization=== | ||
| + | For the 2009 iGEM competition, British_Columbia characterized <partinfo>I13453</partinfo> in the context of a [https://parts.igem.org/PBAD_Promoter_Family pBAD promoter family]. For the results of this characterization, see [[Part:BBa_I13453:British_Columbia|here]]. | ||
| + | |||
| + | For the 2010 iGEM competition, Tec-Monterrey characterized <partinfo>I13453</partinfo> again, using a construct of GFP reporter after the pBad promoter. The transfer function was modeled with a Hill equation. | ||
| + | <!-- --> | ||
| + | [[Image:pbadwt.png|center|frame|Figure 1. Transfer function of <partinfo>BBa_I13453</partinfo>. Points represent individual measurements. The line is of a Hill equation fitted to our data.]] | ||
| + | <center> | ||
| + | (d[GFP]/dt)/OD<sub>600</sub> = C+A*X<sup>n</sup>/(X<sup>n</sup>+K<sup>n</sup>) | ||
| + | {|{{Table}} | ||
| + | !Experiment | ||
| + | !Characteristic | ||
| + | !Value | ||
| + | |- | ||
| + | |rowspan="4"|[[#Transfer Function|'''Transfer Function''']] | ||
| + | |''Basal rate (C)'' | ||
| + | |1 d[GFP]/dt | ||
| + | |- | ||
| + | |''Gain (A)'' | ||
| + | |36 d[GFP]/dt | ||
| + | |- | ||
| + | |''Hill coefficient (n)'' | ||
| + | |2.16 | ||
| + | |- | ||
| + | |''Switch Point (K)'' | ||
| + | |2.8 [ara] (µM) | ||
| + | |||
| + | |- | ||
| + | |} | ||
| + | </center> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
| Line 22: | Line 79: | ||
<partinfo>BBa_I13453 parameters</partinfo> | <partinfo>BBa_I13453 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | |||
| + | ===MetA Knockout Complementation by inducing arabinose promoter by British Columbia iGEM 2012=== | ||
| + | |||
| + | |||
| + | This part consists of an arabinose promoter, a strong RBS, and the MetA coding gene. It is able to complement a metA knockout, and the growth rate appears to be proportional to the amount of arabinose that is added. | ||
| + | |||
| + | <p align=center> https://static.igem.org/mediawiki/parts/thumb/d/d3/MetA_complementation_no_fluor.png/800px-MetA_complementation_no_fluor.png</p> | ||
| + | |||
| + | |||
| + | To generate this graph we cultured an E. coli MetA knockout transformed with this part in M9 minimal media with the indicated concentrations of arabinose, and measured the OD600 every 15 minutes. | ||
| + | |||
| + | ===TrpA Knockout Complementation by inducing arabinose promoter by British Columbia iGEM 2012=== | ||
| + | |||
| + | This part consists of an arabinose promoter, a strong RBS, and the TrpA coding gene. It is able to complement a metA knockout, and the growth rate appears to be proportional to the amount of arabinose that is added. | ||
| + | |||
| + | https://static.igem.org/mediawiki/parts/thumb/0/04/TrpA_Complementation_Test_No_fluor.png/800px-TrpA_Complementation_Test_No_fluor.png | ||
| + | |||
| + | To generate this graph we cultured an E. coli TrpA knockout transformed with this part in M9 minimal media with the indicated concentrations of arabinose, and measured the OD600 every 15 minutes. See <partinfo>BBa_K804009 for negative controls for this part as well as linked fluorescent data. | ||
Latest revision as of 06:31, 14 September 2024
Pbad promoter
PBad promoter from I0500 without AraC.
Usage and Biology
Has been used as a second promoter in a system containing BBa_I0500 (PBad+AraC). In this system, it showed behavior qualitatively indistinguishable from the BBa_I0500 copy of PBad. Has not been tested independent of AraC. A second part, BBa_I13458, should allow decoupling of PBad and AraC.
See this OpenWetWare article on [http://openwetware.org/wiki/Titratable_control_of_pBAD_and_lac_promoters_in_individual_E._coli_cells#pBAD_promotersOpenWetWare pBAD and lac promoters] for additional usage and biology information
2024 Squirrel-CHN supplementary applications
Usage and Biology
First, we induced the expression of red fluorescent protein using the arabinose operon to verify its functionality in E. coli DH5α. Then, our team used the arabinose operon to induce the expression of the bacteriolytic proteins T4 holin and T4 lysozyme, with BBa_B0015 as the terminator. We recombined the fragments into the plasmid pET23b and transformed the constructed plasmid into E. coli DH5α.

Fig 1.genetic circuit diagram
Potential application directions
Firstly, we performed sequence amplification of the arabinose operon, bacteriolytic protein T4 holin, and T4 lysozyme.

Fig 2.Agarose gel electrophoresis to verify the sequence amplification
Finally, we introduced the suicide system into E. coli DH5α. The results indicated that under induction with 0.5 mM/L arabinose, the OD600 of the bacterial culture containing the arabinose operon suicide system was significantly lower than that of the control, proving the effectiveness of the suicide system.

Fig 3.Verification of the Arabinose Operon Suicide System(A) Fluorescence/OD600 ratio of red fluorescent protein expression induced by different concentrations of arabinose operon(B) Growth of E. coli DH5α under 0.5 mM/L arabinose
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 125
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 65
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
For the 2009 iGEM competition, British_Columbia characterized BBa_I13453 in the context of a pBAD promoter family. For the results of this characterization, see here.
For the 2010 iGEM competition, Tec-Monterrey characterized BBa_I13453 again, using a construct of GFP reporter after the pBad promoter. The transfer function was modeled with a Hill equation.
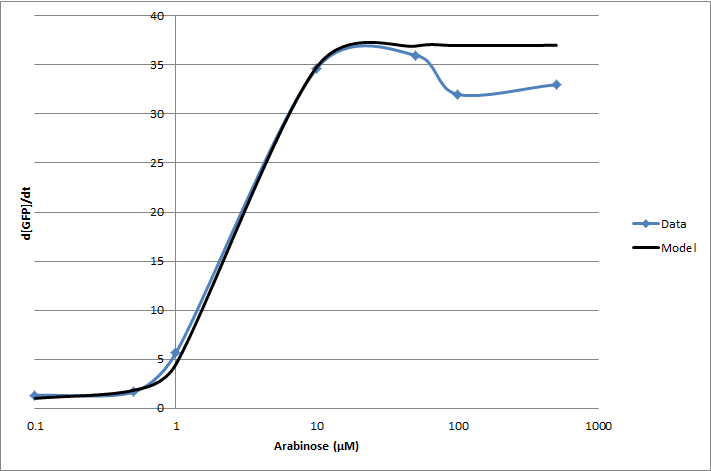
(d[GFP]/dt)/OD600 = C+A*Xn/(Xn+Kn)
| Experiment | Characteristic | Value |
|---|---|---|
| Transfer Function | Basal rate (C) | 1 d[GFP]/dt |
| Gain (A) | 36 d[GFP]/dt | |
| Hill coefficient (n) | 2.16 | |
| Switch Point (K) | 2.8 [ara] (µM) |
MetA Knockout Complementation by inducing arabinose promoter by British Columbia iGEM 2012
This part consists of an arabinose promoter, a strong RBS, and the MetA coding gene. It is able to complement a metA knockout, and the growth rate appears to be proportional to the amount of arabinose that is added.

To generate this graph we cultured an E. coli MetA knockout transformed with this part in M9 minimal media with the indicated concentrations of arabinose, and measured the OD600 every 15 minutes.
TrpA Knockout Complementation by inducing arabinose promoter by British Columbia iGEM 2012
This part consists of an arabinose promoter, a strong RBS, and the TrpA coding gene. It is able to complement a metA knockout, and the growth rate appears to be proportional to the amount of arabinose that is added.

To generate this graph we cultured an E. coli TrpA knockout transformed with this part in M9 minimal media with the indicated concentrations of arabinose, and measured the OD600 every 15 minutes. See BBa_K804009 for negative controls for this part as well as linked fluorescent data. Not understood
