Difference between revisions of "Part:BBa K771004"
(→Membrane Anchor 3 and Membrane Anchor 4) |
AleAlejandro (Talk | contribs) (→Characterization) |
||
| (15 intermediate revisions by 3 users not shown) | |||
| Line 29: | Line 29: | ||
===Membrane Anchor 3 and Membrane Anchor 4=== | ===Membrane Anchor 3 and Membrane Anchor 4=== | ||
| − | [[Image:12SJTU_splitegfp34. | + | [[Image:12SJTU_splitegfp34.png|400px|center]] |
| − | We fused split EGFP 1 and 2 to Membrane Anchor 4 and Membrane Anchor 3 to test whether Membrane Anchor 3 and Membrane Anchor 4 could constitutively aggregate. Proteins in control group are not expected to aggregate like in experimental group. | + | [[Image:12SJTU Anchor GFP.jpg|450px|center]] |
| + | |||
| + | We fused split EGFP 1 and 2 to Membrane Anchor 4 and Membrane Anchor 3 to test whether Membrane Anchor 3 and Membrane Anchor 4 could constitutively aggregate. Proteins in control group are not expected to aggregate like in experimental group.We coexpressed proteins in experimental group and control group respectively in ''E.coli''. After induction for 6 hours, bacteria samples were taken for fluorescence observationThe result shows that Membrane Anchor 3 and Membrane Anchor 4 could constitutively aggregate through PDZ domain and ligand. | ||
<br\><br\><br\> | <br\><br\><br\> | ||
===Membrane Anchor 4 and Membrane Anchor 5 without VVD=== | ===Membrane Anchor 4 and Membrane Anchor 5 without VVD=== | ||
| − | [[Image:12SJTU_splitegfp45. | + | [[Image:12SJTU_splitegfp45.png|400px|center]] |
| − | [[Image: | + | [[Image:12SJTU Standard GFP 2.jpg|400px|center]] |
| − | + | We fused split EGFP 1 and 2 to Membrane Anchor 4 and Membrane Anchor 5 without VVD([https://parts.igem.org/wiki/index.php?title=Part:BBa_K771008 BBa_K771008]) to test whether Membrane Anchor 4 and Membrane Anchor 5 without VVD could constitutively aggregate. Proteins in control group are not expected to aggregate like in experimental group.We coexpressed proteins in experimental group and control group respectively in ''E.coli''. After induction for 6 hours, bacteria samples were taken for fluorescence observationThe result shows that Membrane Anchor 4 and Membrane Anchor 5 without VVD could constitutively aggregate through GBD domain and ligand. | |
==Application== | ==Application== | ||
Latest revision as of 01:31, 3 October 2012
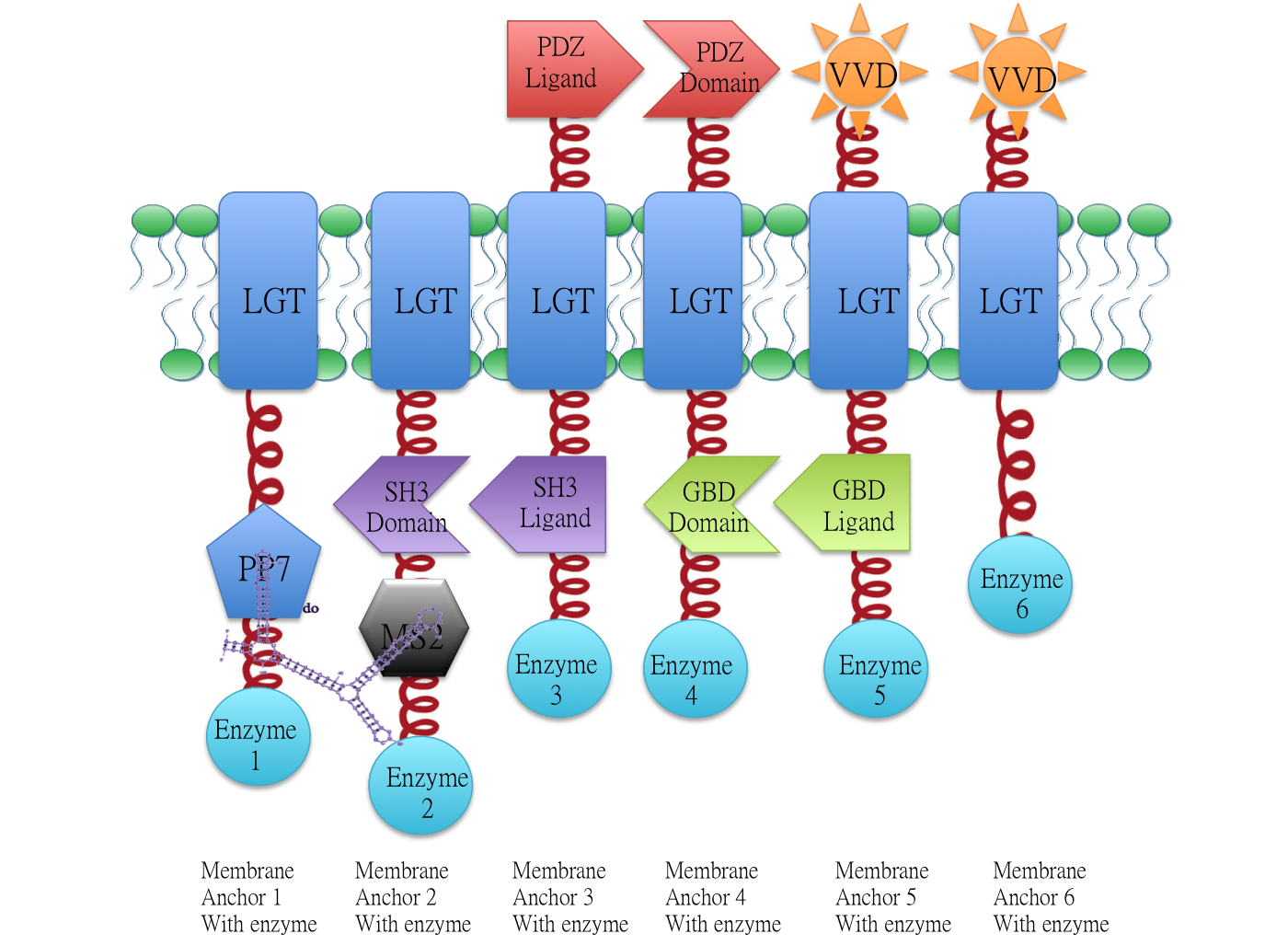
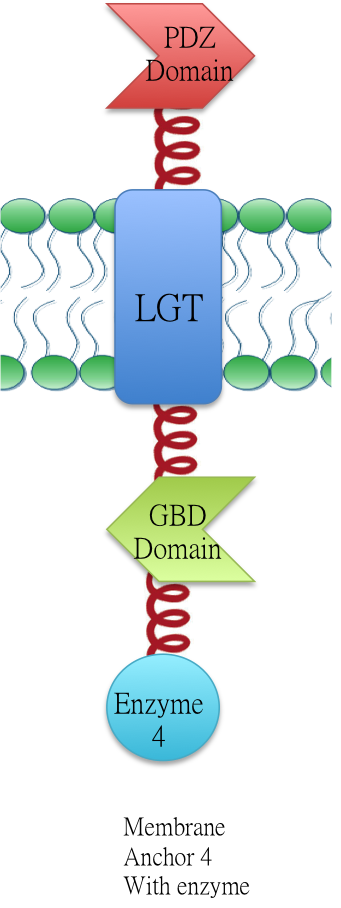
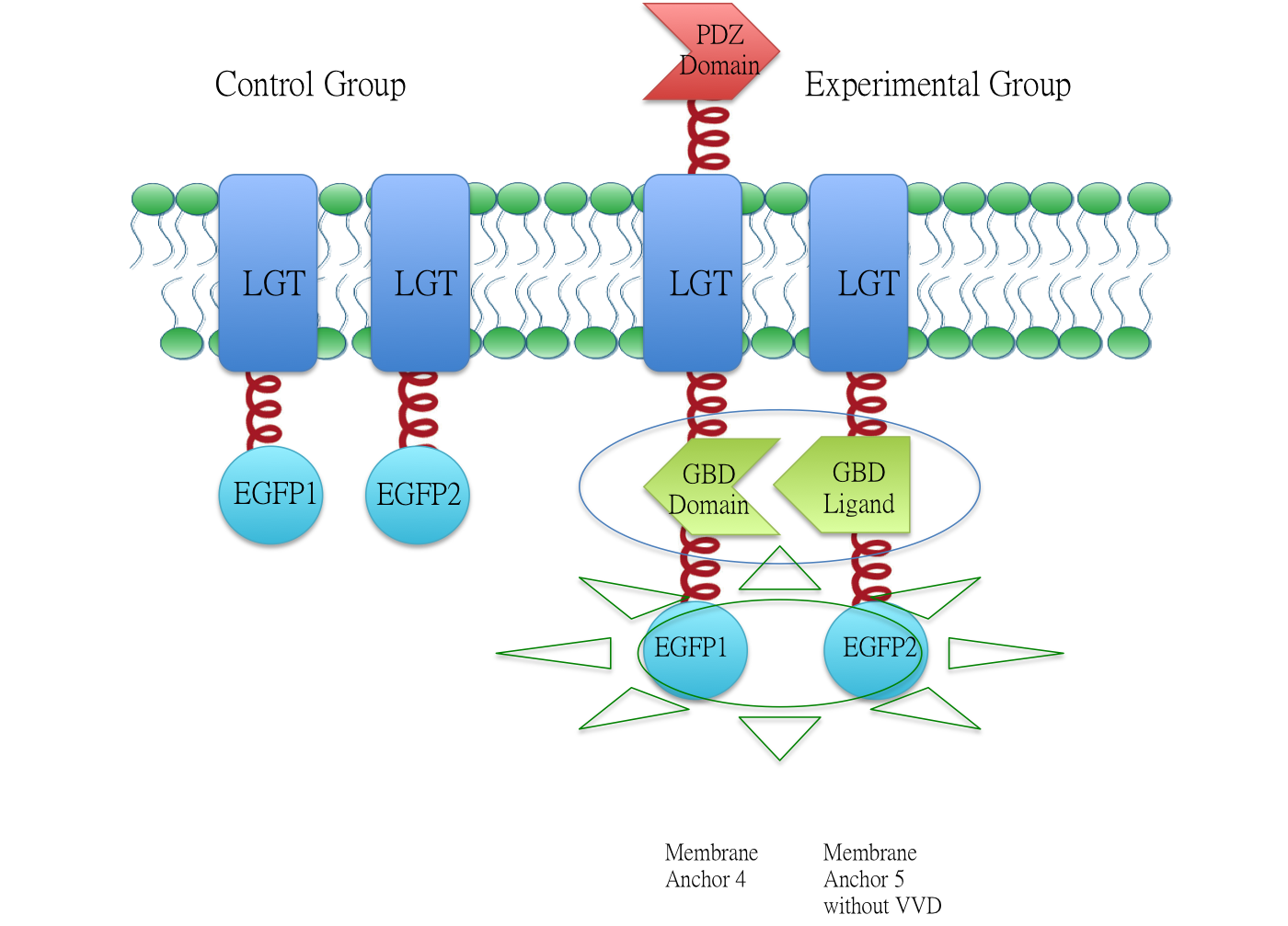
Membrane Anchor 4 : ssDsbA-PDZ Domain-LGT-GBD Domain
Membrane anchor 4, which consists of interacting PDZ domain(BBa_K771103), GBD domain(BBa_K771105) and membrane protein Lgt(BBa_K771102). Membrane Anchor 4 is proved to constitutively aggregate with Membrane Anchor 3 and 5(with and without VVD).
Overview: Membrane Scaffold System
Demonstration of the whole Membrane Scaffold device. Membrane Anchor 2, 3, 4, 5 consitutively aggregate while Membrane Anchor 1 and 6 only aggregate with constitutive cluster (Membrane Anchor 2, 3, 4, 5) when certain signal is present.
Backgroud
Constitutive Aggregation
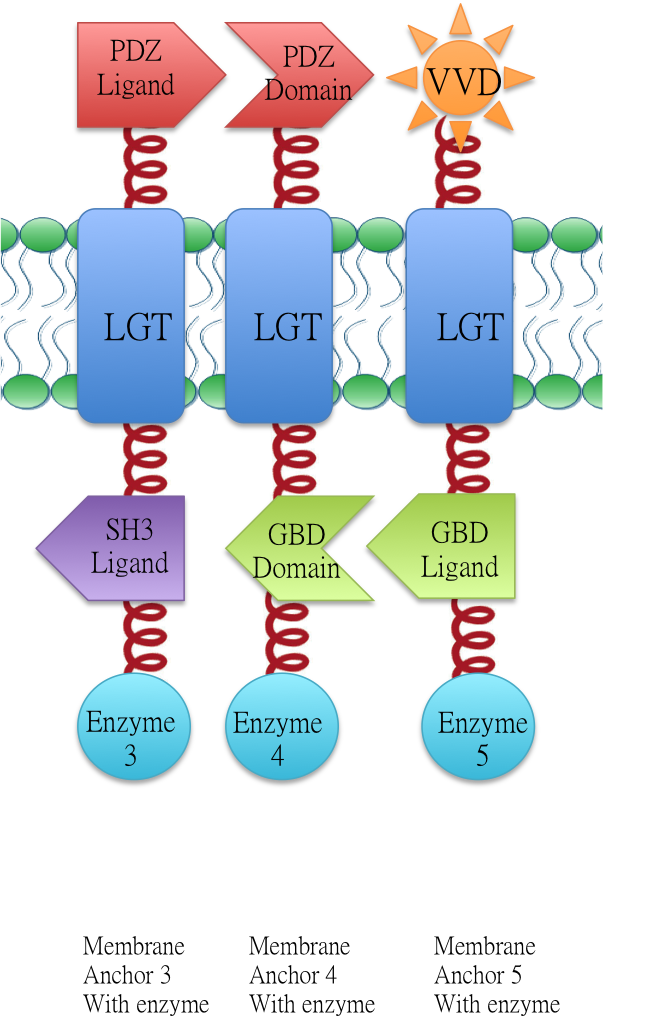
Membrane Anchor 3 (BBa_K771003)and Membrane Anchor 4 constitutively aggregate through PDZ domain(BBa_K771103) and PDZ ligand (BBa_K771104).
Membrane Anchor 4 and Membrane Anchor 5 (with and without VVD) constitutively aggregate through GBD domain(BBa_K771105) and GBD ligand (BBa_K771106).
Design
Membrane Anchor 4 could constitutively aggregate with Membrane Anchor 3 and Membrane Anchor 5 through interacting domain and ligand.
Characterization
To testify the constitutive aggregation of Membrane Anchor 3, 4 and 5, we conducted Fluorescence Complementation Assay.
In fluorescence complementation assay, proteins that are postulated to interact are fused to unfolded complementary fragments of EGFP and expressed in E.coli. Interaction of these proteins will bring the fluorescent fragments within proximity, allowing the reporter protein to reform in its native three-dimensional structure and emit its fluorescent signal. EGFP was split into two halves, named 1EGFP(BBa_K771113) and 2EGFP(BBa_K771114) respectively. If there is interaction between two proteins which were fused with 1EGFP and 2EGFP, it is expected that fluorescence should be observed. Otherwise, no fluorescence could be observed.
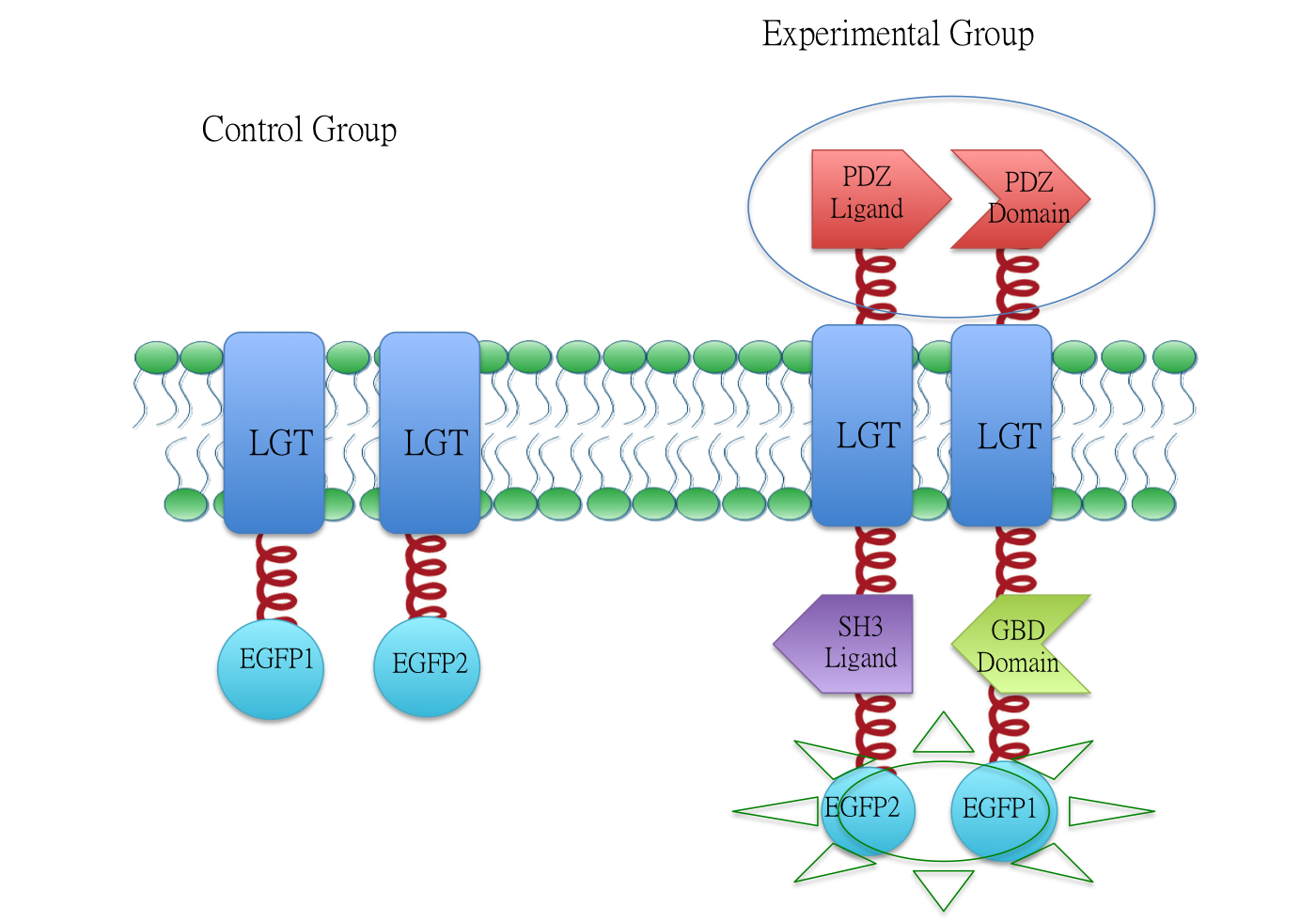
Membrane Anchor 3 and Membrane Anchor 4
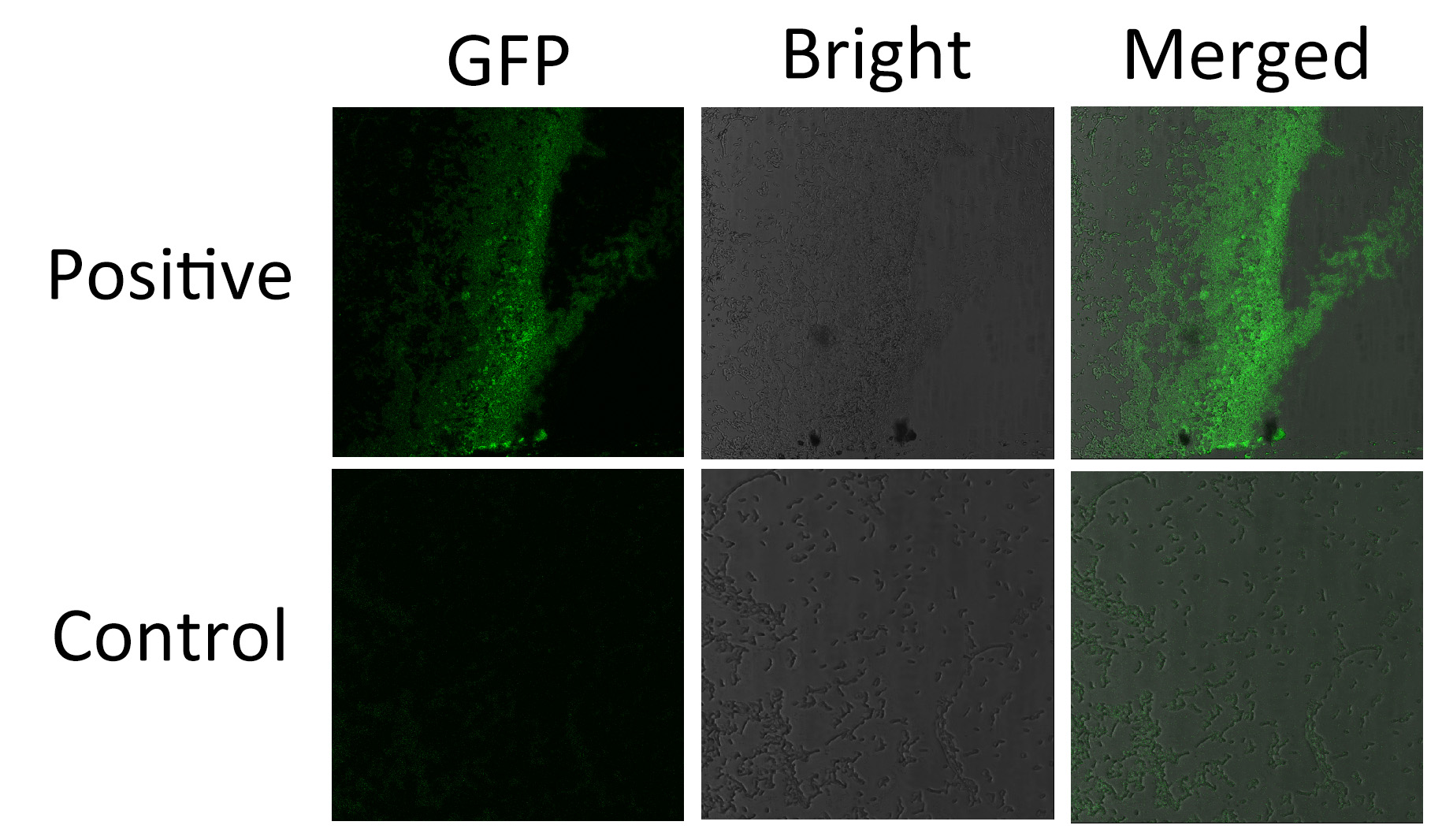
We fused split EGFP 1 and 2 to Membrane Anchor 4 and Membrane Anchor 3 to test whether Membrane Anchor 3 and Membrane Anchor 4 could constitutively aggregate. Proteins in control group are not expected to aggregate like in experimental group.We coexpressed proteins in experimental group and control group respectively in E.coli. After induction for 6 hours, bacteria samples were taken for fluorescence observationThe result shows that Membrane Anchor 3 and Membrane Anchor 4 could constitutively aggregate through PDZ domain and ligand.
<br\><br\><br\>
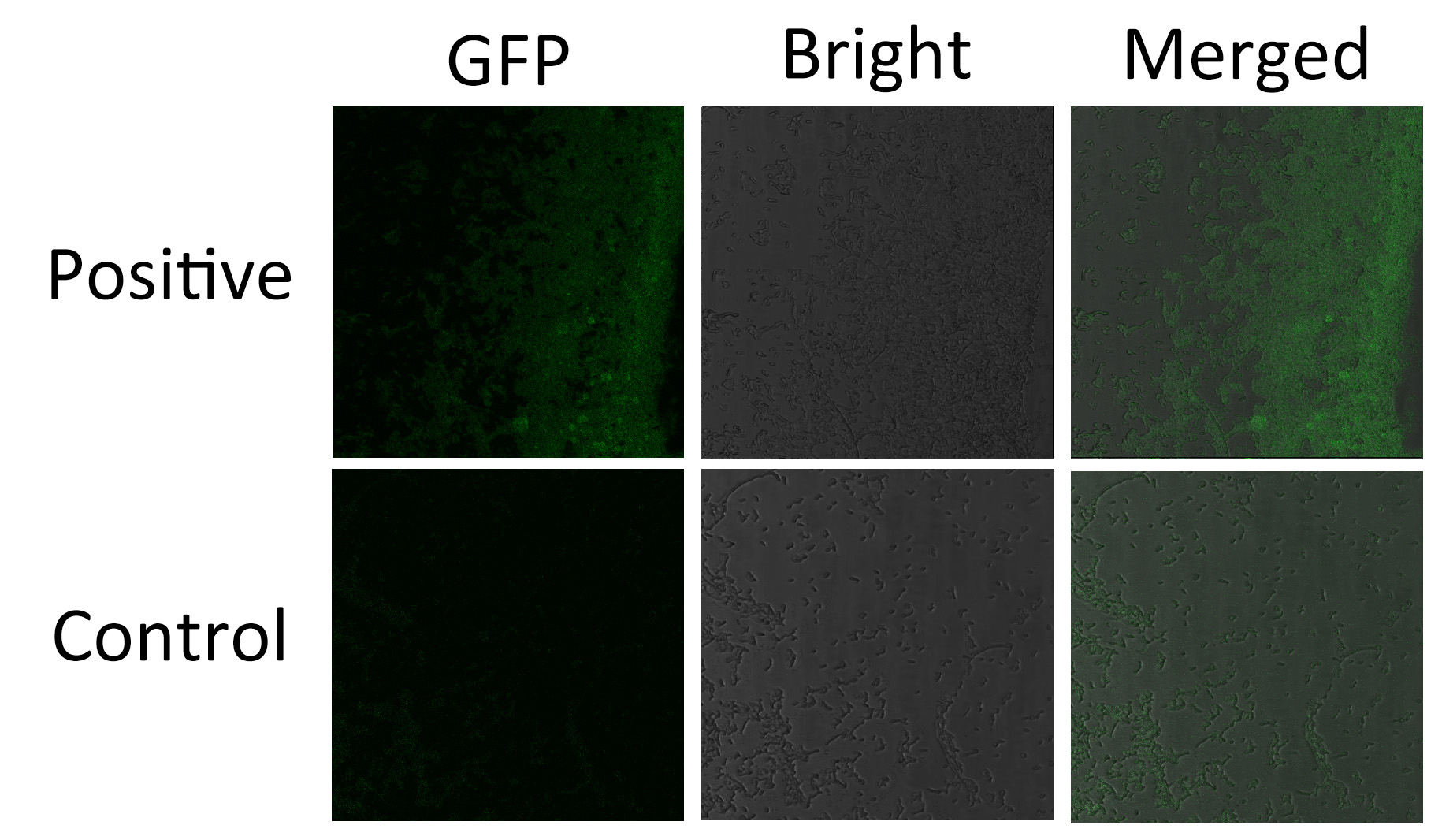
Membrane Anchor 4 and Membrane Anchor 5 without VVD
We fused split EGFP 1 and 2 to Membrane Anchor 4 and Membrane Anchor 5 without VVD(BBa_K771008) to test whether Membrane Anchor 4 and Membrane Anchor 5 without VVD could constitutively aggregate. Proteins in control group are not expected to aggregate like in experimental group.We coexpressed proteins in experimental group and control group respectively in E.coli. After induction for 6 hours, bacteria samples were taken for fluorescence observationThe result shows that Membrane Anchor 4 and Membrane Anchor 5 without VVD could constitutively aggregate through GBD domain and ligand.
Application
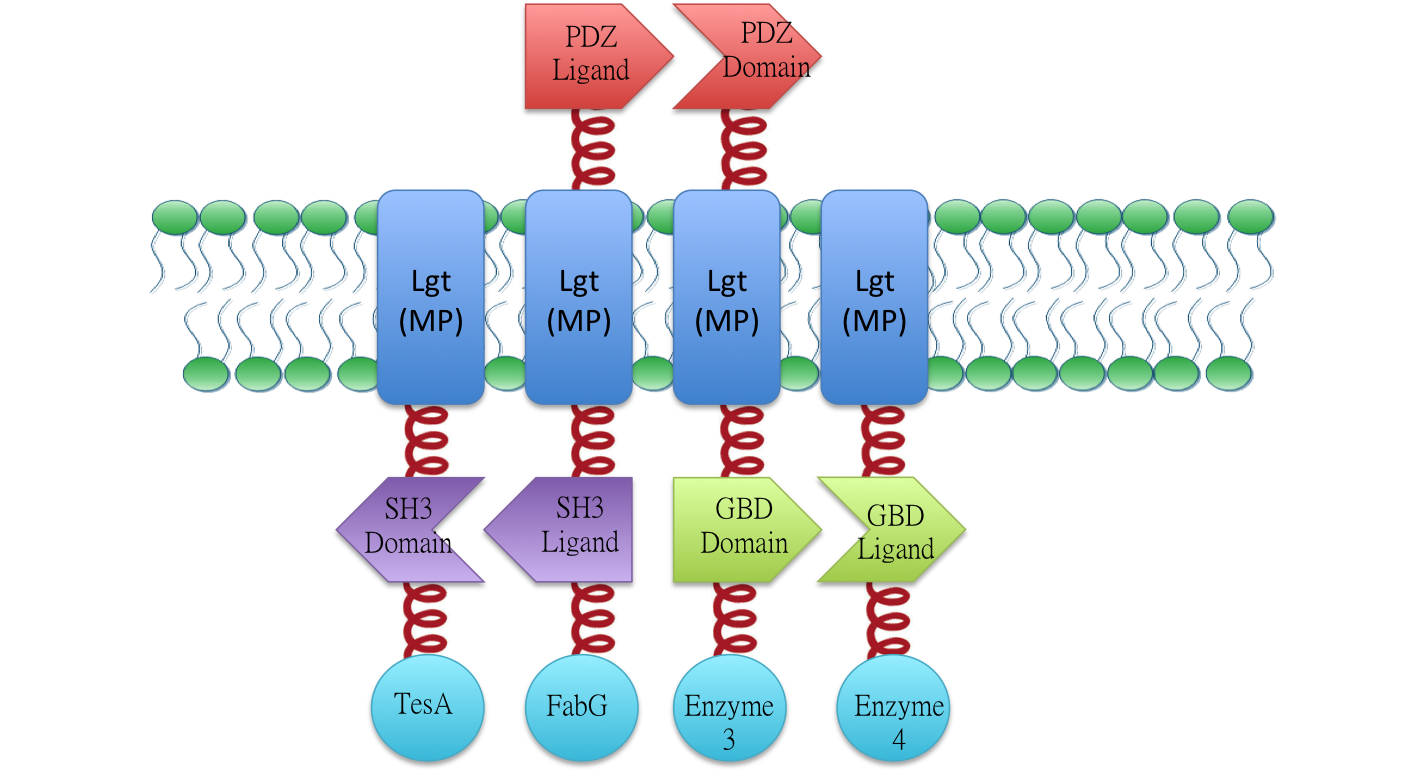
We recruired fatty acid biosynthetic pathway involving TesA(BBa_K771301), FabG(BBa_K771302)) , FabI(BBa_K771303), FabZ(BBa_K771304) to put our membrane scaffold system into practice. Fatty Acid productivity is enhanced by 24 fold.
We constructed TesA with Membrane Anchor 2 (without MS2) (BBa_K771305),FabG with Membrane Anchor 3,(BBa_K771306), FabZ with Membrane Anchor 4 (BBa_K771307), FabI with Membrane Anchor 4(without VVD )(BBa_K771308). Click into each part for more infomation.
Related Biobrick
<br\>Membrabe Anchor 1([BBa_K771001]) <br\>Membrabe Anchor 2([BBa_K771002]) <br\>Membrabe Anchor 3([BBa_K771003]) <br\>Membrabe Anchor 5([BBa_K771005]) <br\>Membrabe Anchor 6([BBa_K771006])
Reference
Dueber, J. E., G. C. Wu, et al. (2009). "Synthetic protein scaffolds provide modular control over metabolic flux." Nature biotechnology 27(8): 753-759.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 115
Illegal AgeI site found at 1174 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 345
Illegal BsaI site found at 795