Difference between revisions of "Part:BBa K792006"
Manugimenez (Talk | contribs) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K792006 short</partinfo> | <partinfo>BBa_K792006 short</partinfo> | ||
| − | '''TrpZipper''' is a small peptide that folds into a beta-hairpin secondary structure. The indole rings of the Trp form a hydrophobic core. The protein is water soluble and | + | '''TrpZipper''' is a small peptide that folds into a beta-hairpin secondary structure. The indole rings of the Trp form a hydrophobic core. The protein is water soluble and monomeric. |
| + | |||
| + | {| class="wikitable" width="170px" | ||
| + | |- | ||
| + | | [[image:TrpZipper2-structure.jpg | 170px]] | ||
| + | |- | ||
| + | |''Molecular structure of TrpZipper2 as determined by NMR (Cochran et al 2001).'' | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| style="width:100%" | ||
| + | |- | ||
| + | | style="background: yellow; width:11%" rowspan="2"| '''See also: ''' | ||
| + | | style="background: #D8D8D8" | Another water soluble Trp-rich peptide <partinfo>K792008</partinfo> | ||
| + | |} | ||
| − | + | {| style="width:100%; background: #CEE3F6" | |
| − | + | |- | |
| + | |This parts was originally built to be used in [http://2012.igem.org/Team:Buenos_Aires iGEM BsAs 2012 project] to implement a ''cross-feeding'' regulatory system between two different yeast strains with specific auxotrophy. [http://2012.igem.org/Team:Buenos_Aires Visit our wiki] to read details about the designing process and implementation details of our system. Also feel free to contact us for any question regarding this part. | ||
| + | |[[Image:Igem.bsas.png | 100px]] | ||
| + | |} | ||
| − | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K792006 SequenceAndFeatures</partinfo> | <partinfo>BBa_K792006 SequenceAndFeatures</partinfo> | ||
Latest revision as of 03:32, 29 October 2012
Tryptophan rich peptide (TrpZipper2)
TrpZipper is a small peptide that folds into a beta-hairpin secondary structure. The indole rings of the Trp form a hydrophobic core. The protein is water soluble and monomeric.
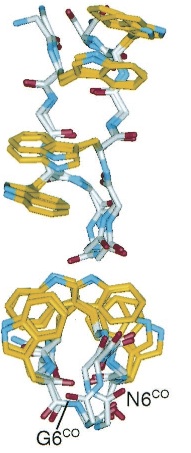
|
| Molecular structure of TrpZipper2 as determined by NMR (Cochran et al 2001). |
| See also: | Another water soluble Trp-rich peptide BBa_K792008 |
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]

