Difference between revisions of "Part:BBa K823036"
| (6 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
cMyc-tag with RBS in [[Help:Assembly_standard_25|Freiburg standard]]. | cMyc-tag with RBS in [[Help:Assembly_standard_25|Freiburg standard]]. | ||
| − | + | Find out more about the design of our [https://static.igem.org/mediawiki/parts/b/b9/LMU-Munich_2012_Our_Freiburg_standard_FusionPrefix.pdf prefix with ribosome binding site]. | |
prefix:GAATTCCGCGGCCGCTTCTAGATAAGGAGGAACTACTATGGCCGGC | prefix:GAATTCCGCGGCCGCTTCTAGATAAGGAGGAACTACTATGGCCGGC | ||
| Line 10: | Line 10: | ||
suffix:ACCGGTTAATACTAGTAGCGGCCGCTGCAGT | suffix:ACCGGTTAATACTAGTAGCGGCCGCTGCAGT | ||
| + | |||
| + | <p align="justify"> | ||
| + | The cMyc-tag is a tag derived from the cMyc gene product. Antibodies were derived from the immunisation with synthetic peptides from the cMyc sequence [http://mcb.asm.org/content/5/12/3610.short Mol. Cell. Biol. 5,3610-3616]). The aminoacid sequence is <b>EQKLISEEDL</b>.</p> | ||
This is a part created by the LMU-Munich 2012 team. We added five tags to the registry, all in the Freiburg standard for N-and C-terminal fusions: | This is a part created by the LMU-Munich 2012 team. We added five tags to the registry, all in the Freiburg standard for N-and C-terminal fusions: | ||
| Line 26: | Line 29: | ||
| − | Visit our project page for more usefull parts of our [http://2012.igem.org/Team:LMU-Munich/Bacillus_BioBricks '''''BacillusB'''''io'''B'''rick'''B'''ox]. | + | Visit our project page for more usefull parts of our [http://2012.igem.org/Team:LMU-Munich/Bacillus_BioBricks '''''BacillusB'''''io'''B'''rick'''B'''ox]. This part was also evaluated in the publication [http://www.jbioleng.org/content/7/1/29 The ''Bacillus'' BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with ''Bacillus subtilis''] by Radeck ''et al.''. |
| + | |||
| + | ===Evaluation=== | ||
| + | <p align="justify"> | ||
| + | All 5 epitope tags were fused C- and N-terminally to GFP using the NgoMIV and AgeI restriction sites. These constructs were expressed in ''Bacillus subtils'' using [https://parts.igem.org/Part:BBa_K823026 pSB<sub>Bs</sub>0K-P<sub>spac</sub>]. This vector did not need to be induced by IPTG due to a premature stop codon in the lacI gene. | ||
| + | |||
| + | |||
| + | </p> | ||
| + | |||
| + | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:left;" | ||
| + | | style="width: 70%;background-color: #EBFCE4;" | | ||
| + | {| | ||
| + | |[[Image:LMU-Western_Blot_Tags.png|400px|center]] | ||
| + | |- | ||
| + | | style="width: 70%;background-color: #EBFCE4;" | | ||
| + | {| style="color:black;" cellpadding="0" width="100%" cellspacing="0" border="0" align="center" style="text-align:center;" | ||
| + | |style="width: 70%;background-color: #EBFCE4;" | | ||
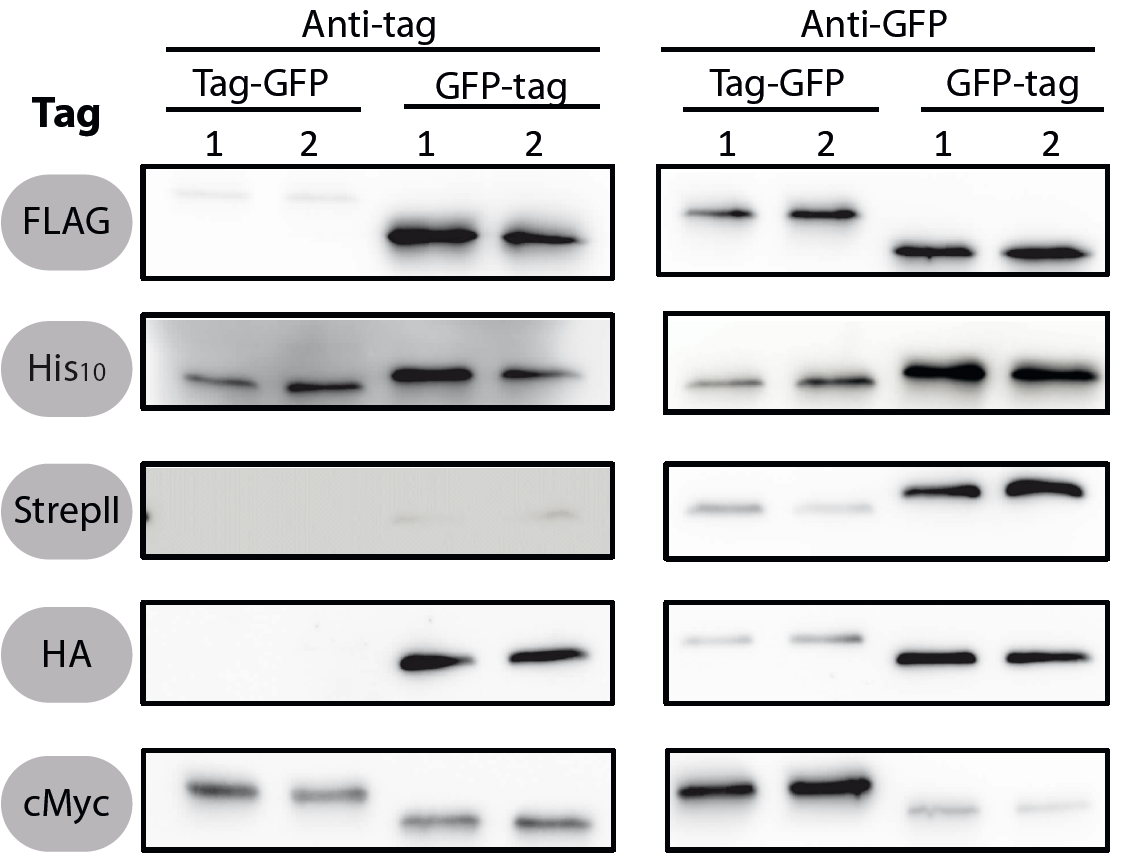
| + | <font color="#000000"; size="2"><p align="justify"> Fig. 1: Western blots of N- and C-terminal fusions of each tag to GFP, using the strains TMB1920 (Flag-gfp), TMB1921 (gfp-Flag), TMB1922 (HA-gfp), TMB1923 (gfp-HA), TMB1924 (cMyc-gfp), TMB1925 (gfp-cMyc), TMB1926 (His-gfp), TMB1927 (gfp-His), TMB1928 (StrepII-gfp) and TMB1929 (gfp-StrepII). For each construct, two independent clones were tested with epitope tag- and GFP-specific antibodies as a positive control. | ||
| + | |||
| + | |} | ||
| + | |} | ||
| + | |} | ||
| + | |||
| + | ===Methods=== | ||
| + | |||
| + | |||
| + | To verify the functionality of the epitope tags, Western blot analyses of the strains TMB1920-TMB1929 were performed. LB medium (15 ml) was inoculated 1:100 from overnight culture and grown at 37°C and 200 rpm to OD600 ~ 0.5. Of this, 10 ml were harvested by centrifugation (8000 × g, 5 min) and the pellets stored at -20°C. Pellets were resuspended in 1 ml disruption buffer (50 mM Tris–HCl pH 7.5, 100 mM NaCl) and lysed by sonication. Samples (12 μl of lysate) were loaded per lane on two 12.5% SDS-polyacrylamide gels and SDS-PAGE was performed according standard procedure [60]. One gel was stained with colloidal coomassie, the other one was used for protein transfer to a PVDF membrane (Merck Millipore, Billerica, MA, USA) by submerged blotting procedure (Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA)). After protein transfer, the membranes were treated with the following antibodies and conditions. Detailed protocols can be found [http://www.jbioleng.org/content/7/1/29/suppl/S3 here]. | ||
| + | |||
| + | |||
| + | ''GFP'' | ||
| + | |||
| + | Probing with primary antibodies takes place with rabbit anti-GFP antibodies (1:3000, Epitomics, No. 1533). Horseradish-peroxidase (HRP)-conjugated anti-rabbit antibodies (1:2000, Promega, W401B) were used as secondary antibody. Hybridization of both antibodies was carried out in Blotto-buffer (2.5% (w/v) skim milk powder, 1 × TBS (50 mM Tris–HCl pH 7.6, 0.15 M NaCl)). | ||
| + | |||
| + | |||
| + | ''cMyc'' | ||
| + | |||
| + | Rabbit anti-Myc (1:2000, Abcan, ab9106) in TBS, 0.05% (w/v) Tween20, 5% (w/v) skim milk powder and anti-rabbit-HRP (1:2000, Promega, W401B) in Blotto-buffer were used. | ||
| + | |||
| + | Chemiluminescence signals were detected after addition of the HRP-substrate Ace Glow (Peqlab, Erlangen, Germany) using a Fusion<sup>TM</sup> imaging system (Peqlab). | ||
| + | </p> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | |||
| + | ==Using the myc tag with mammalian cells== | ||
| + | We utilized this part in the composite part [https://parts.igem.org/Part:BBa_K4359006 (BBa_K4359006)]. Given that the sequence of the myc-tag is derived from the sequence of c-myc, we hypothesized that antibodies against the myc-tag may cross-react with c-myc or other components of mammalian cell lines. We tested this hypothesis by probing the whole cell lysate of SK-BR-3, a breast cancer cell line. While we were unable to ascertain whether the signal detected was from c-myc, we concluded that the anti-myc tag antibody cross-reacted with some other component(s) of the cell lysate. | ||
| + | [[File:Skbr3blot.png|100px|thumb|center| Western blot of SK-BR-3 cell lysate. The membrane was probed with monoclonal anti-myc tag antibody. C-myc is 49 kDa but is known to exhibit aberrant migration in gel electrophoresis. | ||
| + | ]] | ||
| + | We do not recommend usage with mammalian systems. | ||
| − | + | == Team HKUST 2022 == | |
| − | == | + | Team HKUST 2022 utilize this part as a backup for protein verification. |
<!-- --> | <!-- --> | ||
Latest revision as of 14:28, 12 October 2022
cMyc-tag (Freiburg standard+RBS)
cMyc-tag with RBS in Freiburg standard.
Find out more about the design of our prefix with ribosome binding site.
prefix:GAATTCCGCGGCCGCTTCTAGATAAGGAGGAACTACTATGGCCGGC
suffix:ACCGGTTAATACTAGTAGCGGCCGCTGCAGT
The cMyc-tag is a tag derived from the cMyc gene product. Antibodies were derived from the immunisation with synthetic peptides from the cMyc sequence [http://mcb.asm.org/content/5/12/3610.short Mol. Cell. Biol. 5,3610-3616]). The aminoacid sequence is EQKLISEEDL.
This is a part created by the LMU-Munich 2012 team. We added five tags to the registry, all in the Freiburg standard for N-and C-terminal fusions:
- cMyc - tag
Visit our project page for more usefull parts of our [http://2012.igem.org/Team:LMU-Munich/Bacillus_BioBricks BacillusBioBrickBox]. This part was also evaluated in the publication [http://www.jbioleng.org/content/7/1/29 The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis] by Radeck et al..
Evaluation
All 5 epitope tags were fused C- and N-terminally to GFP using the NgoMIV and AgeI restriction sites. These constructs were expressed in Bacillus subtils using pSBBs0K-Pspac. This vector did not need to be induced by IPTG due to a premature stop codon in the lacI gene.
|
Methods
To verify the functionality of the epitope tags, Western blot analyses of the strains TMB1920-TMB1929 were performed. LB medium (15 ml) was inoculated 1:100 from overnight culture and grown at 37°C and 200 rpm to OD600 ~ 0.5. Of this, 10 ml were harvested by centrifugation (8000 × g, 5 min) and the pellets stored at -20°C. Pellets were resuspended in 1 ml disruption buffer (50 mM Tris–HCl pH 7.5, 100 mM NaCl) and lysed by sonication. Samples (12 μl of lysate) were loaded per lane on two 12.5% SDS-polyacrylamide gels and SDS-PAGE was performed according standard procedure [60]. One gel was stained with colloidal coomassie, the other one was used for protein transfer to a PVDF membrane (Merck Millipore, Billerica, MA, USA) by submerged blotting procedure (Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA)). After protein transfer, the membranes were treated with the following antibodies and conditions. Detailed protocols can be found [http://www.jbioleng.org/content/7/1/29/suppl/S3 here].
GFP
Probing with primary antibodies takes place with rabbit anti-GFP antibodies (1:3000, Epitomics, No. 1533). Horseradish-peroxidase (HRP)-conjugated anti-rabbit antibodies (1:2000, Promega, W401B) were used as secondary antibody. Hybridization of both antibodies was carried out in Blotto-buffer (2.5% (w/v) skim milk powder, 1 × TBS (50 mM Tris–HCl pH 7.6, 0.15 M NaCl)).
cMyc
Rabbit anti-Myc (1:2000, Abcan, ab9106) in TBS, 0.05% (w/v) Tween20, 5% (w/v) skim milk powder and anti-rabbit-HRP (1:2000, Promega, W401B) in Blotto-buffer were used.
Chemiluminescence signals were detected after addition of the HRP-substrate Ace Glow (Peqlab, Erlangen, Germany) using a FusionTM imaging system (Peqlab).
Using the myc tag with mammalian cells
We utilized this part in the composite part (BBa_K4359006). Given that the sequence of the myc-tag is derived from the sequence of c-myc, we hypothesized that antibodies against the myc-tag may cross-react with c-myc or other components of mammalian cell lines. We tested this hypothesis by probing the whole cell lysate of SK-BR-3, a breast cancer cell line. While we were unable to ascertain whether the signal detected was from c-myc, we concluded that the anti-myc tag antibody cross-reacted with some other component(s) of the cell lysate.
We do not recommend usage with mammalian systems.
Team HKUST 2022
Team HKUST 2022 utilize this part as a backup for protein verification.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 24
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]