Difference between revisions of "Part:BBa K658003"
(rollbackEdits.php mass rollback) |
|||
| (7 intermediate revisions by 4 users not shown) | |||
| Line 4: | Line 4: | ||
This device is designed to build a programmed bacterial death circuit, which is based on the quorum sensing system of Vibrio fischeri. This device has the ability to maintain cell density of the bacteria population at a relatively low value compared with bacteria without this circuit and extends the stable stage. RBS0.6 was used to regulate the expression of killer protein ccdB. | This device is designed to build a programmed bacterial death circuit, which is based on the quorum sensing system of Vibrio fischeri. This device has the ability to maintain cell density of the bacteria population at a relatively low value compared with bacteria without this circuit and extends the stable stage. RBS0.6 was used to regulate the expression of killer protein ccdB. | ||
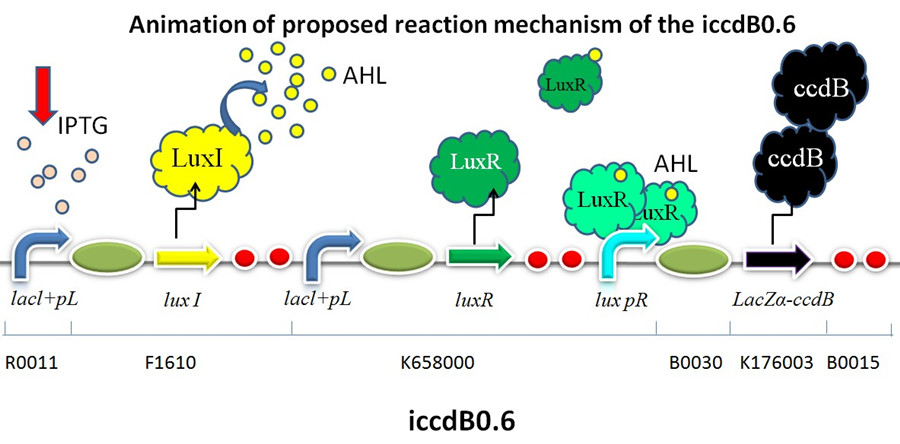
| − | + | [[Image:XMU_China_31beta.jpg|left|Figure 1 Animation of proposed reaction mechanism of iccdB0.6|frame|Figure 1 Animation of proposed reaction mechanism of iccdB0.6.]] | |
| − | |||
iGEM Team XMU-China generated a series of population-control devices based on iccdB0.6 (BBa_K658003) by applying mutated promoters lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007) and lux pR-3/5(BBa_K658008) in this circuit instead of lux pR (BBa_R0062). These devices program the steady-state cell density maintaining at different levels. | iGEM Team XMU-China generated a series of population-control devices based on iccdB0.6 (BBa_K658003) by applying mutated promoters lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007) and lux pR-3/5(BBa_K658008) in this circuit instead of lux pR (BBa_R0062). These devices program the steady-state cell density maintaining at different levels. | ||
| Line 20: | Line 19: | ||
The performance of iccdB0.6 was tested by measuring its steady-state cell density and comparing it with other population control devices. | The performance of iccdB0.6 was tested by measuring its steady-state cell density and comparing it with other population control devices. | ||
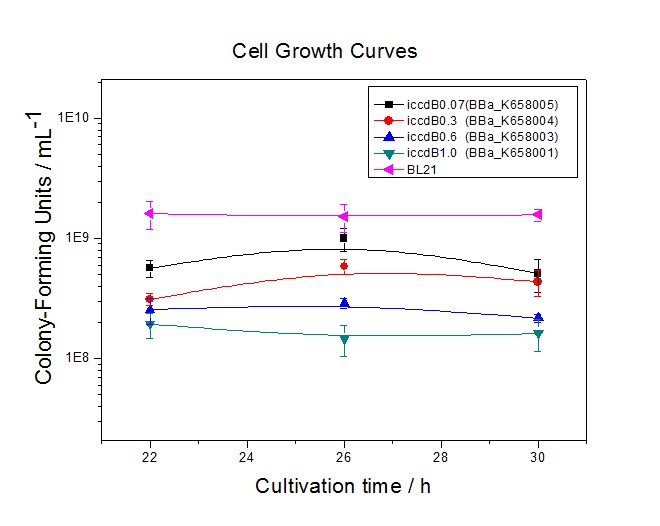
| − | + | [[Image:XMU_China_32.jpg|left|Figure 2 Experimentally measured densities of BL21’s cells without population-control circuit and BL21’s cells with four population-control circuits respectively at steady state|frame|Figure 2 Experimentally measured densities of BL21’s cells without population-control circuit and BL21’s cells with four population-control circuits respectively at steady state.]] | |
| − | |||
| − | |||
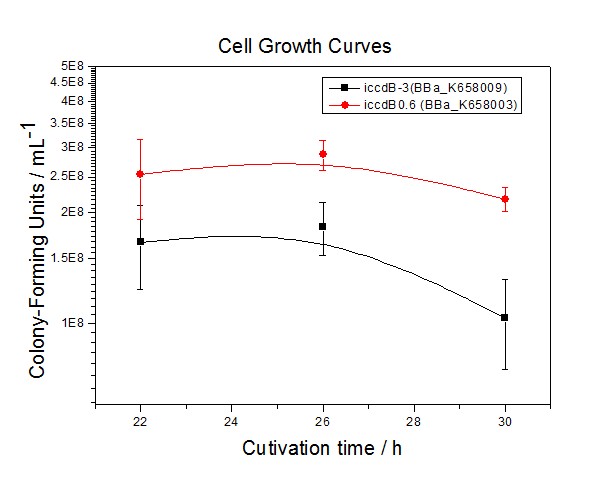
| − | Figure 3 Experimentally measured steady-state cell density of iccdB0.6(BBa_K658003) and iccdB-3 (BBa_K658009) | + | [[Image:XMU_China_33.jpg|left|Figure 3 Experimentally measured steady-state cell density of iccdB0.6(BBa_K658003) and iccdB-3 (BBa_K658009)|frame|Figure 3 Experimentally measured steady-state cell density of iccdB0.6(BBa_K658003) and iccdB-3 (BBa_K658009).]] |
| − | |||
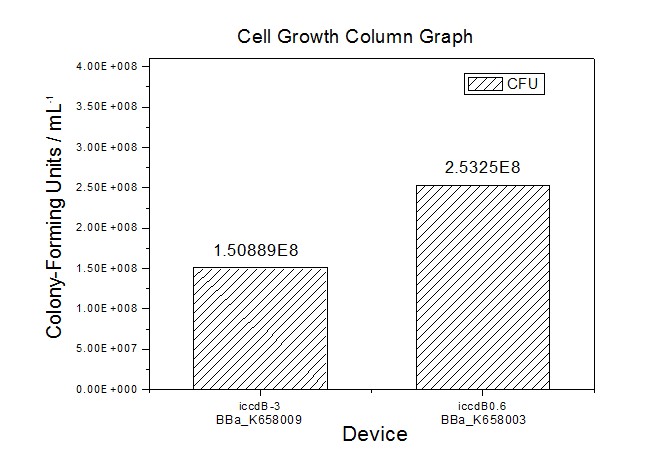
| − | Figure 4 Average of experimentally measured cell densities of BL21’s cells with iccdB0.6 (BBa_K658003) and its mutant iccdB-3 (BBa_K658009) | + | [[Image:XMU_China_34.jpg|left|Figure 4 Average of experimentally measured cell densities of BL21’s cells with iccdB0.6 (BBa_K658003) and its mutant iccdB-3 (BBa_K658009)|frame|Figure 4 Average of experimentally measured cell densities of BL21’s cells with iccdB0.6 (BBa_K658003) and its mutant iccdB-3 (BBa_K658009).]] |
| + | <html> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | |||
Figure 3 and Figure 4 illustrate that the population-control device iccdB-3 programs a relatively lower steady-state cell density compared with iccdB0.6. This matched the result of the test on four lux pR promoters’ strength in our IR-GFP device (BBa_K658016). The strength of lux pR promoters were defined as follows: | Figure 3 and Figure 4 illustrate that the population-control device iccdB-3 programs a relatively lower steady-state cell density compared with iccdB0.6. This matched the result of the test on four lux pR promoters’ strength in our IR-GFP device (BBa_K658016). The strength of lux pR promoters were defined as follows: | ||
| − | + | [[Image:XMU_China_35.jpg|left|Figure 5 Efficiency of promoter lux pR (R0062) and its 3 mutants|frame|Figure 5 Efficiency of promoter lux pR (R0062) and its 3 mutants.]] | |
| + | <html> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/4/41/XMU_China_block.jpg"> | ||
| + | </html> | ||
| + | |||
| − | |||
As is shown in figure 5, promoter lux pR-3 has the highest strength of the four. It is probable that mutation at position 3 lowers the threshold for the binding reaction between LuxR/AHL protein complex and promoter lux pR, which starts the Quorum Sensing system at a relatively earlier period with a lower cell density compared with circuits regulated by wild type promoter lux pR (BBa_R0062). | As is shown in figure 5, promoter lux pR-3 has the highest strength of the four. It is probable that mutation at position 3 lowers the threshold for the binding reaction between LuxR/AHL protein complex and promoter lux pR, which starts the Quorum Sensing system at a relatively earlier period with a lower cell density compared with circuits regulated by wild type promoter lux pR (BBa_R0062). | ||
| Line 42: | Line 47: | ||
Once the QS system is started, downstream killer protein expresses. The viable cell density reaches a steady state when cell growth rate equals to its death rate. Generally, steady-state cell density seems to fluctuate at the cell density when QS is started. Thus, the higher strength a promoter has, the earlier the population-control device is started, leading to a lower steady-state cell density. | Once the QS system is started, downstream killer protein expresses. The viable cell density reaches a steady state when cell growth rate equals to its death rate. Generally, steady-state cell density seems to fluctuate at the cell density when QS is started. Thus, the higher strength a promoter has, the earlier the population-control device is started, leading to a lower steady-state cell density. | ||
| − | The earlier the QS system is started, the more GFP might be produced, leading to a higher fluorescence intensity at steady state. | + | The earlier the QS system is started, the more GFP might be produced, leading to a higher fluorescence intensity at steady state. |
| − | + | ||
== '''References''' == | == '''References''' == | ||
| − | [1] Koch B, Liljefors T, Persson T , Nielsen J, Kjelleberg S, Givskov M. The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors[J].Microbiology , 2005, 151 (11): 3589-3602. | + | [1] You L, Cox RS, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing[J]. Nature, 2004, 428(6985): 868-871. |
| + | |||
| + | [2] Baldwin T, Devine JH, Heckel, RC, Lin, JW, Shadel GS. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence[J]. Journal of Bioluminescence and Chemiluminescence, 1989, 4(1): 326-341. | ||
| + | |||
| + | [3] Koch B, Liljefors T, Persson T , Nielsen J, Kjelleberg S, Givskov M. The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors[J].Microbiology , 2005, 151 (11): 3589-3602. | ||
| − | [ | + | [4] Kumari A, Pasini P, Deo S. K., Flomenhoft D, Shashidhar S, Daunert S. Biosensing Systems for the Detection of Bacterial Quorum Signaling Molecules[J]. Analytical Chemistry,2005, 78 (22): 7603–7609. |
| − | [ | + | [5] Eberhard A, Burlingame A. L, Eberhard C, Kenyon G. L, Nealson K. H,Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase[J]. Biochemistry ,1981,20 (9): 2444–2449. |
| − | [ | + | [6] http://scholarworks.umass.edu/dissertations/AAI3397713/ |
| − | [ | + | [7] Gerdes K. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress[J]. J. Bacteriol,2000,182 (3): 561–721. |
| − | [ | + | [8]Philippe B,Martine C.Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes[J].Molecular Biology,1992,226(3):735-745. |
| − | [ | + | [9]Devine J.H,Shadel G.S,Baldwin T.O. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proceedings of the National Academy of Sciences.1989.86(15):5688-5692. |
| − | [ | + | [10] http://www.uniprot.org/uniprot/P12747 |
Latest revision as of 16:18, 10 May 2013
a bacteria population-control device with RBS0.6 driven by lacl+pL
This device is designed to build a programmed bacterial death circuit, which is based on the quorum sensing system of Vibrio fischeri. This device has the ability to maintain cell density of the bacteria population at a relatively low value compared with bacteria without this circuit and extends the stable stage. RBS0.6 was used to regulate the expression of killer protein ccdB.
iGEM Team XMU-China generated a series of population-control devices based on iccdB0.6 (BBa_K658003) by applying mutated promoters lux pR-3 (BBa_K658006), lux pR-5(BBa_K658007) and lux pR-3/5(BBa_K658008) in this circuit instead of lux pR (BBa_R0062). These devices program the steady-state cell density maintaining at different levels.
Description
This device is made up of three subparts: a LuxI producer, a LuxR producer and a killer protein producer. . The LuxI protein synthesizes a small, diffusible acyl-homoserinelactone (AHL) signaling molecule. The AHL accumulates as the cell density increases. At sufficiently high concentrations, it binds the LuxR, which induces the expression of the killer gene ccdB under the control of a promoter lux pR. Sufficiently high levels of CcdB which is a bacterial toxin that targets DNA gyrase cause cell death. Low cell density doesn’t have the ability to produce sufficient LuxR/AHL complex to activate the promoter lux pR. The programmed death circuit ends and the cell density increases. When the cells reach a certain concentration, the death circuit is restarted. Back and forth, the programmed death is achieved in the dynamic process of growth and death. In that way, the bacteria population is programmed to maintain one certain cell density.
RBS0.6 (BBa_B0030) within the killer protein producer guarantees a moderate expression of LacZα-ccdB fusion preotein. And this is the reason why iccdB0.6 was chosen as a “mother device” to test the performance of mutated lux pR promoters in a population-control circuit.
Performance
The performance of iccdB0.6 was tested by measuring its steady-state cell density and comparing it with other population control devices.

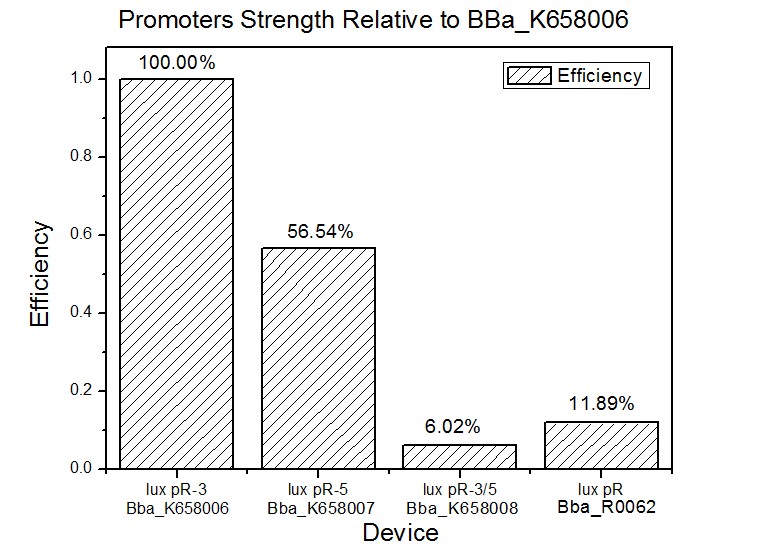
Figure 3 and Figure 4 illustrate that the population-control device iccdB-3 programs a relatively lower steady-state cell density compared with iccdB0.6. This matched the result of the test on four lux pR promoters’ strength in our IR-GFP device (BBa_K658016). The strength of lux pR promoters were defined as follows:

As is shown in figure 5, promoter lux pR-3 has the highest strength of the four. It is probable that mutation at position 3 lowers the threshold for the binding reaction between LuxR/AHL protein complex and promoter lux pR, which starts the Quorum Sensing system at a relatively earlier period with a lower cell density compared with circuits regulated by wild type promoter lux pR (BBa_R0062).
Once the QS system is started, downstream killer protein expresses. The viable cell density reaches a steady state when cell growth rate equals to its death rate. Generally, steady-state cell density seems to fluctuate at the cell density when QS is started. Thus, the higher strength a promoter has, the earlier the population-control device is started, leading to a lower steady-state cell density.
The earlier the QS system is started, the more GFP might be produced, leading to a higher fluorescence intensity at steady state.
References
[1] You L, Cox RS, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing[J]. Nature, 2004, 428(6985): 868-871.
[2] Baldwin T, Devine JH, Heckel, RC, Lin, JW, Shadel GS. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence[J]. Journal of Bioluminescence and Chemiluminescence, 1989, 4(1): 326-341.
[3] Koch B, Liljefors T, Persson T , Nielsen J, Kjelleberg S, Givskov M. The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors[J].Microbiology , 2005, 151 (11): 3589-3602.
[4] Kumari A, Pasini P, Deo S. K., Flomenhoft D, Shashidhar S, Daunert S. Biosensing Systems for the Detection of Bacterial Quorum Signaling Molecules[J]. Analytical Chemistry,2005, 78 (22): 7603–7609.
[5] Eberhard A, Burlingame A. L, Eberhard C, Kenyon G. L, Nealson K. H,Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase[J]. Biochemistry ,1981,20 (9): 2444–2449.
[6] http://scholarworks.umass.edu/dissertations/AAI3397713/
[7] Gerdes K. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress[J]. J. Bacteriol,2000,182 (3): 561–721.
[8]Philippe B,Martine C.Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes[J].Molecular Biology,1992,226(3):735-745.
[9]Devine J.H,Shadel G.S,Baldwin T.O. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proceedings of the National Academy of Sciences.1989.86(15):5688-5692.
[10] http://www.uniprot.org/uniprot/P12747
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 718
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 2347
Illegal BsaI.rc site found at 1874