Difference between revisions of "Part:BBa K525123"
(→Identification and localisation) |
Eschneider (Talk | contribs) m |
||
| (32 intermediate revisions by 9 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K525123 short</partinfo> | <partinfo>BBa_K525123 short</partinfo> | ||
| + | [[Image:Bielefeld-Germany2011-S-Layer-Geometrien.jpg|300px|thumb|right]] | ||
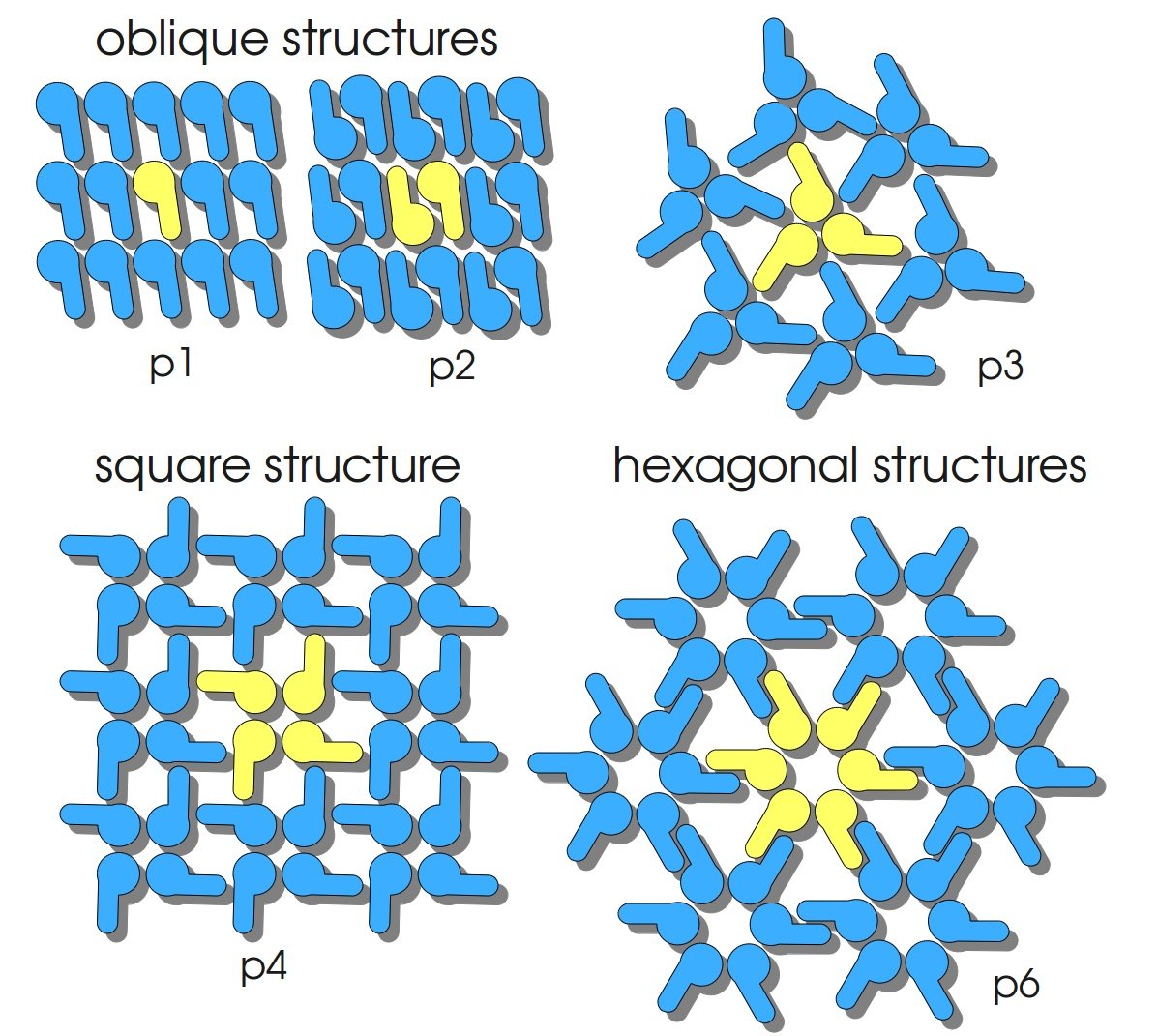
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr ''et al.'', 2007]). | S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr ''et al.'', 2007]). | ||
| Line 18: | Line 19: | ||
!Result | !Result | ||
|- | |- | ||
| − | |rowspan=" | + | |rowspan="4"|[[Part:BBa_K525123#Expression_in_E._coli | Expression (''E. coli'')]] |
| − | + | ||
| − | + | ||
| − | + | ||
|Compatibility | |Compatibility | ||
|''E. coli'' KRX | |''E. coli'' KRX | ||
| Line 33: | Line 31: | ||
|Doubling time (un-/induced) | |Doubling time (un-/induced) | ||
|2.82 h / 7.42 h | |2.82 h / 7.42 h | ||
| + | |- | ||
| + | |rowspan="8"|[[Part:BBa_K525123#Purification_of_SgsE_fusion_protein | Characterization]] | ||
| + | |- | ||
| + | |Number of amino acids | ||
| + | |461 | ||
| + | |- | ||
| + | |Molecular weight | ||
| + | |50.6 kDa | ||
| + | |- | ||
| + | |Theoretical pI | ||
| + | |4.21 | ||
| + | |- | ||
| + | |rowspan="4"|Localization | ||
| + | |cell membrane | ||
| + | |- | ||
| + | |cytoplasm | ||
| + | |- | ||
| + | |not in culture supernatant | ||
| + | |- | ||
| + | |not periplasm | ||
|- | |- | ||
|} | |} | ||
| Line 51: | Line 69: | ||
===Expression in ''E. coli''=== | ===Expression in ''E. coli''=== | ||
| − | For characterization the | + | For characterization, the modified ''cspB'' gene was fused to a monomeric RFP ([https://parts.igem.org/Part:BBa_E1010 BBa_E1010]) using Gibson assembly. |
| − | The CspB|mRFP fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0 | + | The CspB|mRFP fusion protein was overexpressed in ''E. coli'' KRX after induction of a T7 polymerase gene in the KRX's genome by supplementation of 0.1 % L-rhamnose using the autinduction protocol developed by Promega. |
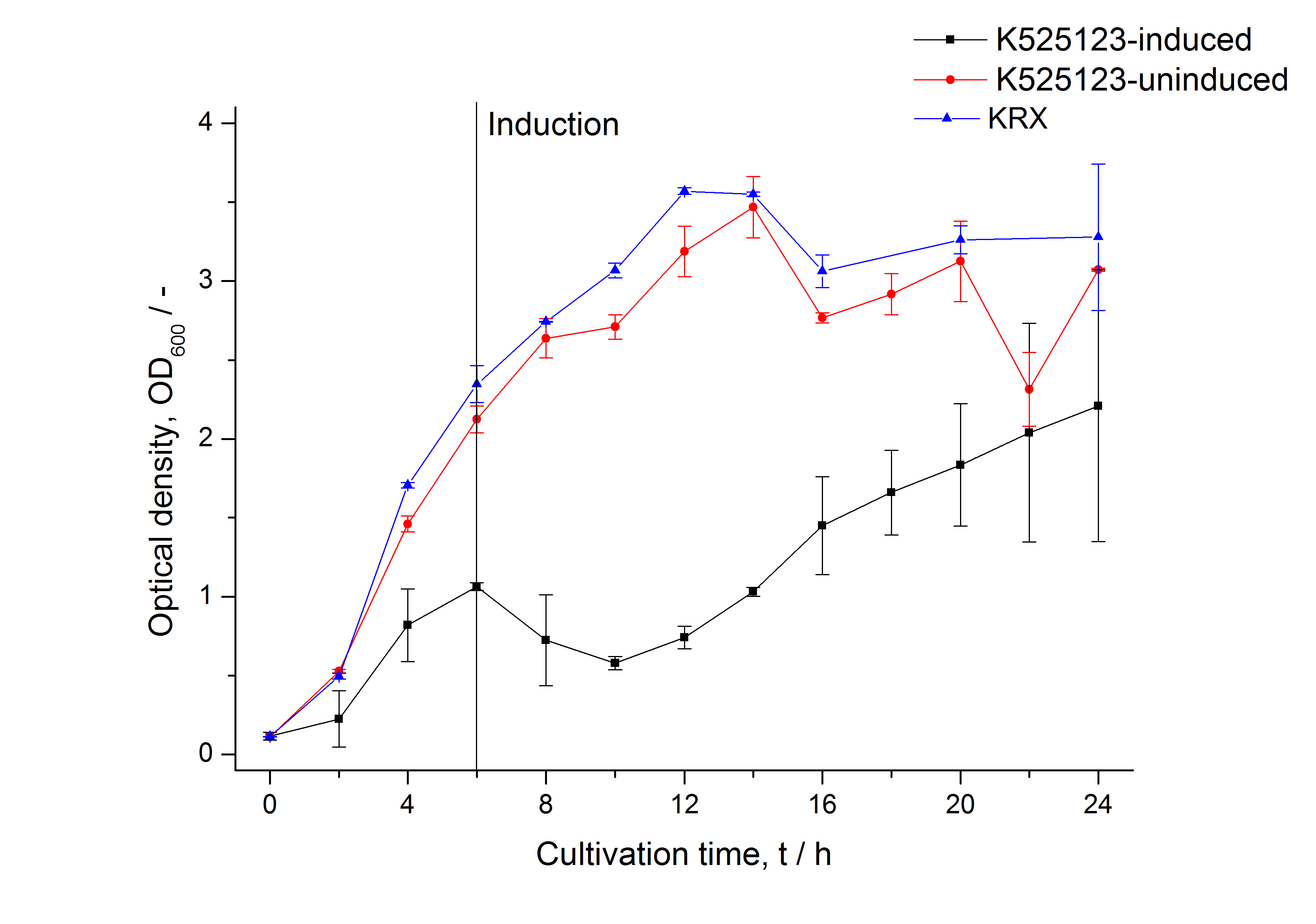
| − | [[Image:Bielefeld_2011_BF3_Growthcurve.png|600px|center|thumb| '''Figure 1: | + | [[Image:Bielefeld_2011_BF3_Growthcurve.png|600px|center|thumb| '''Figure 1: Growth curve of ''E. coli'' KRX expressing the fusion protein of CspB and mRFP with and without induction, cultivated at 37 °C in autoinduction medium with and without inductor, respectively. A curve depicting KRX wildtype is shown for comparsion. After autoinduction at approximately 6 h the OD<sub>600</sub> of the induced <partinfo>K525123</partinfo> visibly drops when compared to the uninduced culture. While the induced culture grow significantly slower than KRX wildtype the uninduced seems to be unaffected.''']] |
| − | [[Image:Bielefeld_2011_BF3_RFU_OD.png|600px|center|thumb| '''Figure 2: RFU to OD<sub>600</sub> ratio of ''E. coli'' KRX expressing the fusion protein of CspB and mRFP with and without induction. A curve depicting KRX wildtype is shown for comparsion.''']] | + | [[Image:Bielefeld_2011_BF3_RFU_OD.png|600px|center|thumb| '''Figure 2: RFU to OD<sub>600</sub> ratio of ''E. coli'' KRX expressing the fusion protein of CspB and mRFP with and without induction. A curve depicting KRX wildtype is shown for comparsion. After induction at approximately 6 h the RFU to OD<sub>600</sub> ratio starts to rise in the induced culture. Compared to the uninduced culture the ratio is roughly eight times higher. Most likely due to basal transcription the RFU to OD<sub>600</sub> ratio of the uninduced culture starts to rise after 12 hours. The KRX wildtype shows no variation in the RFU to OD<sub>600</sub> ratio.''']] |
===Identification and localisation=== | ===Identification and localisation=== | ||
| − | After a cultivation time of 18 h the mRFP|CspB fusion protein | + | After a cultivation time of 18 h the mRFP|CspB fusion protein was localized in ''E. coli'' KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was washed with ddH<sub>2</sub>O. From the other part the periplasm was detached by using an osmotic shock. |
| + | |||
| + | The S-layer fusion protein could not be found in the polyacrylamide gel after a SDS-PAGE of the lysate. This indicated that the fusion protein integrates into the cell membrane with its lipid anchor. For testing this assumption the washed lysate was treated with ionic, nonionic and zwitterionic detergents to release the mRFP|CspB out of the membranes. | ||
| + | |||
| + | The existance of flourescence in the detergent fractions and the not existent fluorescence in the wash fraction confirms the hypothesis of an insertion into the cell membrane (fig. 3). An insertion of these S-layer proteins might stabilize the membrane structure and increase the stability of cells against mechanical and chemical treatment. A stabilization of ''E. coli'' expressing S-lyer proteins was discribed by Lederer ''et al.'', (2010). | ||
| + | |||
| + | Another important fact is that there is actually mRFP fluorescence measurable in such high concentrated detergent solutions. The S-layer seems to stabilize the biologically active conformation of mRFP. The MALDI-TOF analysis of the relevant size range in the polyacrylamid gel approved the existance of the intact fusion protein in all detergent fractions (fig 4). | ||
| + | |||
| + | In comparison with the mRFP fusion protein of [https://parts.igem.org/Part:BBa_K525121 K525121], which has a TAT-sequence, a minor relative fluorescence in all cultivation and detergent fractions was detected (fig. 3). Together with the decreasing RFU/OD<sub>600</sub> after 12 h of cultivation (fig. 2) indicates that the TAT-sequence results in a postive effect on the protein stability. | ||
| + | |||
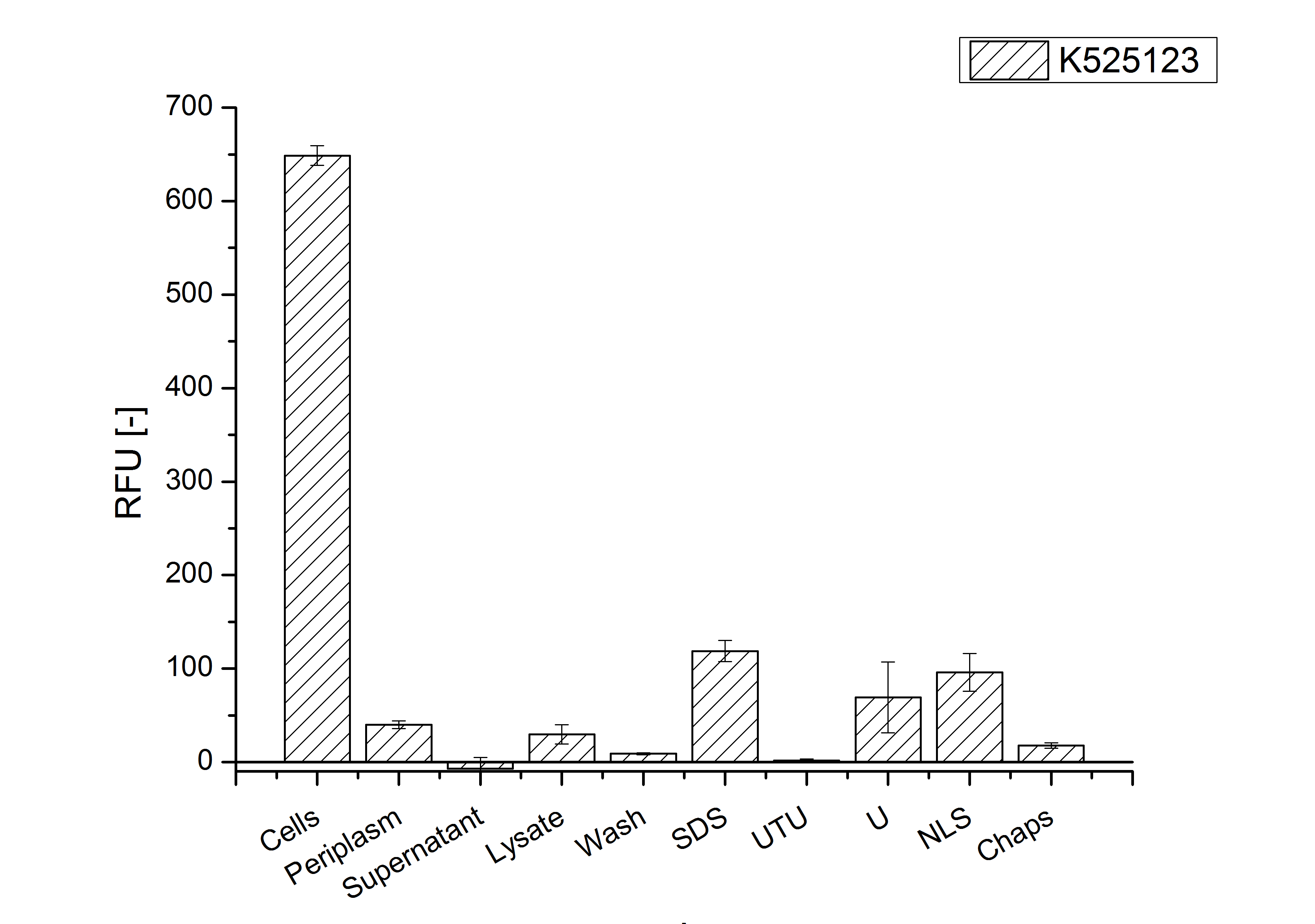
| + | [[Image:Bielefeld 2011 BF3 Purification.png|700px|thumb|center| '''Figure 3: Fluorescence progression of the mRFP[https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)]/CspB fusion protein initiating with the cultivation fractions up to the detergent fractions of the seperate denaturations. Cultivations were carried out in autoinduction medium at 37 ˚C. The cells were mechanically disrupted and the resulting biomass was washed with ddH<sub>2</sub>O and resuspended in the respective detergent. The used detergent acronyms stand for: SDS = 10 % sodium dodecyl sulfate; UTU = 7 M urea and 3 M thiourea; U = 10 M urea; NLS = 10 % n-lauroyl sarcosine; 2 % CHAPS = 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate.''']] | ||
| + | |||
| + | MALDI-TOF analysis was used to identify the location of the fusion protein in different fractions. Fractions of medium supernatant after cultivation (M), periplasmatic isolation (PP), cell lysis (L) and the following wash with ddH<sub>2</sub>O, samples were loaded onto a SDS-PAGE. After comparison with same treated fraction of E. coli KRX all gel bands in a defined size area were cutted out of the gel and analysed with MALDI-TOF. Results are shown in fig. 4. | ||
| + | |||
| + | |||
| + | |||
| + | [[Image:Bielefeld2011_K525123_Gel1.png|900px|thumb| '''Figure 4: MALDI-TOF measurement of the mRFP[https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)]/CspB fusion protein. Data is shown in a SDS-PAGE. Colours show the sequence coverage of MALDI-TOF measurement. On the left side of the gel samples fractions of the fusion protein are shown, on the right side of the gel fractions of an equally treated ''E. coli'' KRX are shown. Measurement was performed with a ultrafleXtremeTM by Bruker Daltonics using the software FlexAnalysis, Biotools and SequenceEditor.''']] | ||
| + | |||
| + | Results show that the fusion protein of mRFP[https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)]|CspB without TAT-sequence and with lipid anchor has only been identified in the lysis fraction. However, in conclusion with absent TAT-sequence, the protein has not been identified in the periplasm and the culture supernatant, respectively. | ||
| + | |||
| + | The influence of other detergents to disintegrate the S-layer fusion protein was tested after disrupting the cells with a ribolyser. The cell pellet was incubated in 10 % (v/v) Sodium dodecyl sulfate (SDS), in 7 M urea and 3 M thiourea (UTU), in 10 M urea (U) in 10 % (v/v) N-lauroyl sarcosine (NLS) and in 2 % CHAPS (C). Samples of the incubations with these detergents were loaded onto a SDS-PAGE prior to measurement with MALDI-TOF (Fig. 5). | ||
| + | |||
| + | |||
| + | [[Image:K525123_BF3_Gel2_A2.png|900px|thumb| '''Figure 5: Influence of diffent detergents on the disintegration of the fusionprotein CspB/mRFP (BBa_E1010) from the cell membrane of ''E. coli'' KRX. The coloured marker shows the sequence coverage of MALDI-TOF measurement. Abbreviations are: SDS (Sodium dodecyl sulfate 10 % (w/v)), NLS ((10 % (w/v) m-lauroyl sarcosine), UTU (3 M thiourea, 7 M urea), U (10 M urea), CH (2% CHAPS 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), Ma Marker (PageRuler TM Prestained Protein Ladder SM0671). On the left half of the gel fractions of the S-layer fusion protein are displayed, on the right half fractions of ''E. coli'' KRX are displayed.''']] | ||
| + | |||
| + | The results of the MALDI-TOF measurement clearly demonstrate that all used detergents are applicable to disintegrate the S-layer fusion proteins from the bacterial cell membrane of ''E. coli''. Fluorescence measurement of fractions treated with the detergents, show significantly different values, indicating that some of the detergents (e.g. 3 M thiourea, 7 M urea) have a strong effect on protein folding. The samples taken from gel lanes of ''E. coli'' KRX show no sequence coverage, therefore not similar proteins are naturally induced in ''E. coli''. | ||
| + | |||
| + | ===Methods=== | ||
| + | |||
| + | '''Expression of S-layer genes in ''E. coli'' ''' | ||
| + | * Chassis: Promega's [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ ''E. coli'' KRX] | ||
| + | |||
| + | * Medium: LB medium supplemented with 20 mg L<sup>-1</sup> chloramphenicol | ||
| + | ** For autoinduction: Cultivations in LB-medium were supplemented with 0.1 % L-rhamnose as inducer and 0.05 % glucose for repression. | ||
| + | |||
| + | |||
| + | '''Measuring of [https://parts.igem.org/Part:BBa_E1010 mRFP]''' | ||
| + | * Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination | ||
| + | * Freeze biological samples at -80 °C for storage, keep cell-free at 4 °C in the dark | ||
| + | * To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination) | ||
| + | * Measure the fluorescence in a platereader (we used a [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]) with following settings: | ||
| + | ** 20 sec orbital shaking (1 mm amplitude with a frequency of 87.6 rpm) | ||
| + | ** Measurement mode: Top | ||
| + | ** Excitation: 584 nm | ||
| + | ** Emission: 620 nm | ||
| + | ** Number of reads: 25 | ||
| + | ** Manual gain: 100 | ||
| + | ** Integration time: 20 µs | ||
| + | |||
| + | |||
| + | '''Tryptic digest of gel lanes for analysis with MALDI-TOF''' | ||
| + | |||
| + | Note: | ||
| + | *Make sure to work under a fume hood. | ||
| + | *Do not work with protective gloves to prevent contamination of your sample with platicizers. | ||
| + | |||
| + | Reaction tubes have to be cleaned with 60% (v/v) CH<sub>3</sub>CN, 0.1% (v/v) TFA. Afterwards the solution has to be removed completely followed by evaporation of the tubes under a fume hood. Alternatively microtiter plates from Greiner® (REF 650161) can be used without washing. | ||
| + | |||
| + | *Cut out the protein lanes of a Coomassie-stained SDS-PAGE using a clean scalpel. Gel parts are transferred to the washed reaction tubes/microtiter plate. If necessary cut the parts to smaller slices. | ||
| + | *Gel slices should be washed two times. Therefore add 200 µL 30% (v/v) acetonitrile in 0.1 M ammonium hydrogen carbonate each time and shake lightly for 10 minutes. Remove supernatant and discard to special waste. | ||
| + | *Dry gel slices at least 30 minutes in a Speedvac. | ||
| + | *Rehydrate gel slices in 15 µL Trypsin-solution followed by short centrifugation. | ||
| + | *Gel slices have to be incubated 30 minutes at room temperature, followed by incubation at 37 °C over night. | ||
| + | *Dry gel slices at least 30 minutes in a Speedvac. | ||
| + | *According to the size of the gel slice, add 5 – 20 µL 50% (v/v) ACN / 0,1% (v/v) TFA. | ||
| + | *Samples can be used for MALDI measurement or stored at -20 °C. | ||
| + | |||
| + | Trypsin-solution: 1 µL Trypsin + 14 µL 10 mM NH<sub>4</sub>HCO<sub>3</sub> | ||
| + | *Therefore solubilize lyophilized Trypsin in 200 µL of provided buffer and incubate for 15 minutes at 30 °C for activation. For further use it can be stored at -20 °C. | ||
| + | |||
| + | |||
| + | '''Preparation and Spotting for analysis of peptides on Bruker AnchorChips''' | ||
| + | |||
| + | *Spot 0,5 – 1 µL sample aliquot | ||
| + | *Add 1 µL HCCA matrix solution to the spotted sample aliquots. Pipet up and down approximately five times to obtain a sufficient mixing. Be careful not to contact the AnchorChip. | ||
| + | Note: Most of the sample solvent needs to be gone in order to achieve a sufficiently low water content. When the matrix solution is added to the previously spotted sample aliquot at a too high water content in the mixture, it will result in undesired crystallization of the matrix outside the anchor spot area. | ||
| + | *Dry the prepared spots at room temperature | ||
| + | *Spot external calibrants on the adjacent calibrant spot positions. Use the calibrant stock solution (Bruker’s “Peptide Calibration Standard II”, Part number #222570), add 125 µL of 0,1% TFA (v/v) in 30% ACN to the vial. Vortex and sonicate the vial. | ||
| + | *Mix the calibrant stock solution in a 1:200 ratio with HCCA matrix and deposit 1 µL of the mixture onto the calibrant spots. | ||
| − | + | '''Release of periplasmic protein fraction from E. coli by cold osmotic shock''' | |
| − | + | *Modified protocol from Neu & Heppel, 1965. | |
| + | *Centrifuge E. coli cell suspension for 5 min at 14000 g (4 °C) to collect the cells. | ||
| + | *Discard the entire supernatant. | ||
| + | *Resuspend the cells ice-cold cell fractionating buffer #1. The resulting volume should be 1/4 of the former suspension volume. | ||
| + | *Incubate for 20 min on ice. Ivert the suspension at regular intervals to counteract sedimentation. | ||
| + | *Centrifuge the cell suspension for 15 min at 14000 g (4 °C). | ||
| + | *Discard the entire supernatant. | ||
| + | *Resuspend the cells ice-cold cell fractionating buffer #2. The resulting volume should be 1/4 of the former suspension volume. | ||
| + | *Incubate for 10 min on ice under regular invertion. | ||
| + | *Centrifuge the cell suspension for 15 min at 14000 g (4 °C). | ||
| + | *Save the supernatant, which contains the periplasmatic proteins. | ||
| + | *If the periplasmatic protein fraction is turbid, re-centrifuge and filter it through a 0.2 µm filter. | ||
| − | + | ===References=== | |
| + | Hansmeier N, Bartels FW, Ros R, Anselmetti D, Tauch A, Pühler A, Kalinowski J (2004) Classification of hyper-variable Corynebacterium glutamicum surface-layer proteins by sequence analyses and atomic force microscopy [http://www.sciencedirect.com/science/article/pii/S016816560400241X J Biotechnol. 26;112(1-2):177-93.] | ||
| − | + | Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full ''FEMS Microbiol Lett'' 267(2):131-144]. | |
| − | |||
<!-- --> | <!-- --> | ||
Latest revision as of 01:26, 9 September 2018
S-layer cspB from Corynebacterium glutamicum with lipid anchor and PT7 and RBS
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
The S-layer of C. glutamicum is characterized by a hexagonal lattice symmetry. Attachment between S-layer and cell wall was found to be due to the hydrophobic carboxy-terminus of the PS2 protein [http://www.sciencedirect.com/science/article/pii/S016816560400241X Hansmeier et al., 2004]).
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Compatibility | E. coli KRX |
| Inductor for expression | L-rhamnose for induction of T7 polymerase | |
| Specific growth rate (un-/induced) | 0.245 h-1 / 0.093 h-1 | |
| Doubling time (un-/induced) | 2.82 h / 7.42 h | |
| Characterization | ||
| Number of amino acids | 461 | |
| Molecular weight | 50.6 kDa | |
| Theoretical pI | 4.21 | |
| Localization | cell membrane | |
| cytoplasm | ||
| not in culture supernatant | ||
| not periplasm |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1334
Illegal XhoI site found at 161
Illegal XhoI site found at 779 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1313
Illegal SapI site found at 560
Illegal SapI site found at 772
Illegal SapI site found at 1320
Expression in E. coli
For characterization, the modified cspB gene was fused to a monomeric RFP (BBa_E1010) using Gibson assembly.
The CspB|mRFP fusion protein was overexpressed in E. coli KRX after induction of a T7 polymerase gene in the KRX's genome by supplementation of 0.1 % L-rhamnose using the autinduction protocol developed by Promega.

Identification and localisation
After a cultivation time of 18 h the mRFP|CspB fusion protein was localized in E. coli KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was washed with ddH2O. From the other part the periplasm was detached by using an osmotic shock.
The S-layer fusion protein could not be found in the polyacrylamide gel after a SDS-PAGE of the lysate. This indicated that the fusion protein integrates into the cell membrane with its lipid anchor. For testing this assumption the washed lysate was treated with ionic, nonionic and zwitterionic detergents to release the mRFP|CspB out of the membranes.
The existance of flourescence in the detergent fractions and the not existent fluorescence in the wash fraction confirms the hypothesis of an insertion into the cell membrane (fig. 3). An insertion of these S-layer proteins might stabilize the membrane structure and increase the stability of cells against mechanical and chemical treatment. A stabilization of E. coli expressing S-lyer proteins was discribed by Lederer et al., (2010).
Another important fact is that there is actually mRFP fluorescence measurable in such high concentrated detergent solutions. The S-layer seems to stabilize the biologically active conformation of mRFP. The MALDI-TOF analysis of the relevant size range in the polyacrylamid gel approved the existance of the intact fusion protein in all detergent fractions (fig 4).
In comparison with the mRFP fusion protein of K525121, which has a TAT-sequence, a minor relative fluorescence in all cultivation and detergent fractions was detected (fig. 3). Together with the decreasing RFU/OD600 after 12 h of cultivation (fig. 2) indicates that the TAT-sequence results in a postive effect on the protein stability.
MALDI-TOF analysis was used to identify the location of the fusion protein in different fractions. Fractions of medium supernatant after cultivation (M), periplasmatic isolation (PP), cell lysis (L) and the following wash with ddH2O, samples were loaded onto a SDS-PAGE. After comparison with same treated fraction of E. coli KRX all gel bands in a defined size area were cutted out of the gel and analysed with MALDI-TOF. Results are shown in fig. 4.
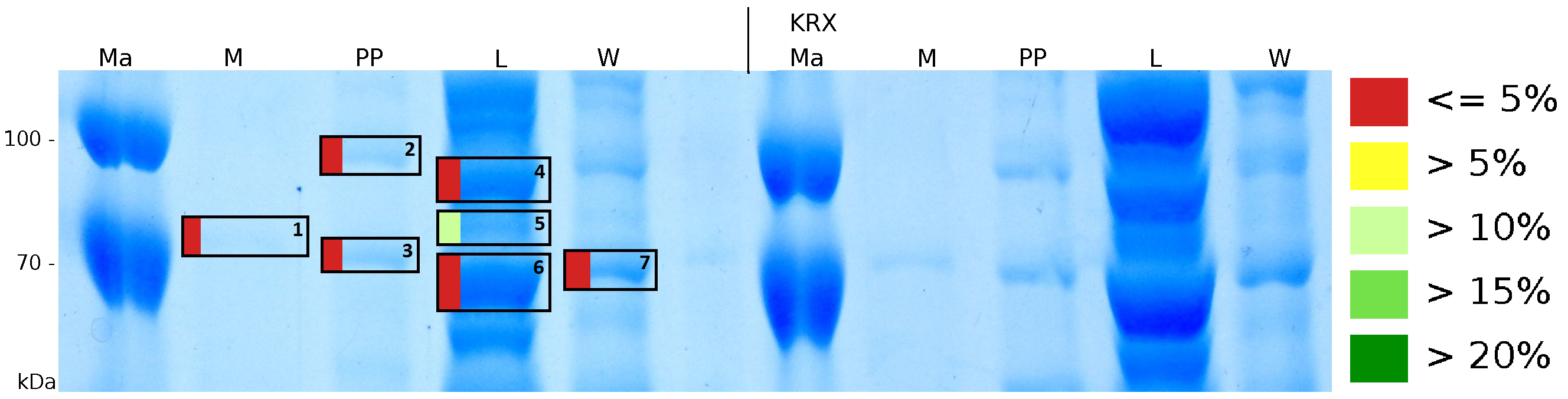
Results show that the fusion protein of mRFP(BBa_E1010)|CspB without TAT-sequence and with lipid anchor has only been identified in the lysis fraction. However, in conclusion with absent TAT-sequence, the protein has not been identified in the periplasm and the culture supernatant, respectively.
The influence of other detergents to disintegrate the S-layer fusion protein was tested after disrupting the cells with a ribolyser. The cell pellet was incubated in 10 % (v/v) Sodium dodecyl sulfate (SDS), in 7 M urea and 3 M thiourea (UTU), in 10 M urea (U) in 10 % (v/v) N-lauroyl sarcosine (NLS) and in 2 % CHAPS (C). Samples of the incubations with these detergents were loaded onto a SDS-PAGE prior to measurement with MALDI-TOF (Fig. 5).
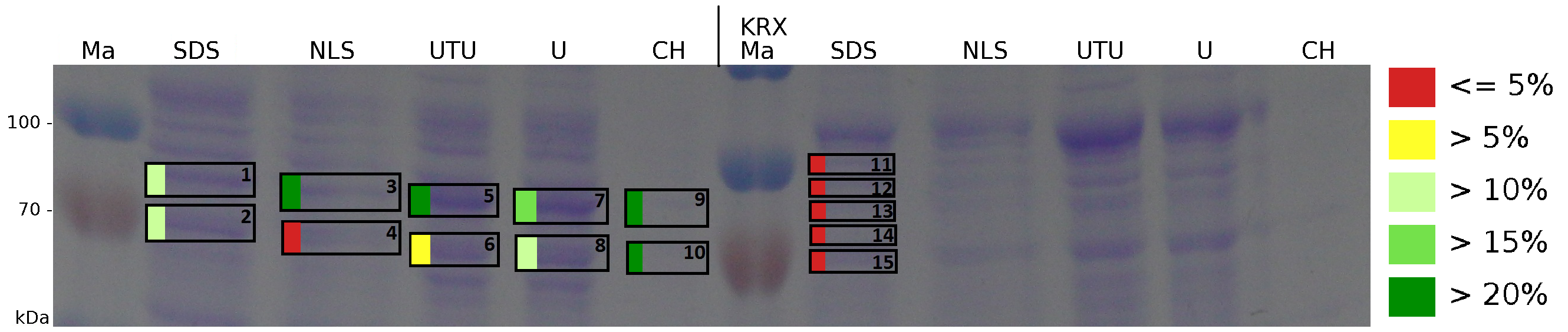
The results of the MALDI-TOF measurement clearly demonstrate that all used detergents are applicable to disintegrate the S-layer fusion proteins from the bacterial cell membrane of E. coli. Fluorescence measurement of fractions treated with the detergents, show significantly different values, indicating that some of the detergents (e.g. 3 M thiourea, 7 M urea) have a strong effect on protein folding. The samples taken from gel lanes of E. coli KRX show no sequence coverage, therefore not similar proteins are naturally induced in E. coli.
Methods
Expression of S-layer genes in E. coli
- Chassis: Promega's [http://www.promega.com/products/cloning-and-dna-markers/cloning-tools-and-competent-cells/bacterial-strains-and-competent-cells/single-step-_krx_-competent-cells/ E. coli KRX]
- Medium: LB medium supplemented with 20 mg L-1 chloramphenicol
- For autoinduction: Cultivations in LB-medium were supplemented with 0.1 % L-rhamnose as inducer and 0.05 % glucose for repression.
Measuring of mRFP
- Take at least 500 µL sample for each measurement (200 µL is needed for one measurement) so you can perform a repeat determination
- Freeze biological samples at -80 °C for storage, keep cell-free at 4 °C in the dark
- To measure the samples thaw at room temperature and fill 200 µL of each sample in one well of a black, flat bottom 96 well microtiter plate (perform at least a repeat determination)
- Measure the fluorescence in a platereader (we used a [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]) with following settings:
- 20 sec orbital shaking (1 mm amplitude with a frequency of 87.6 rpm)
- Measurement mode: Top
- Excitation: 584 nm
- Emission: 620 nm
- Number of reads: 25
- Manual gain: 100
- Integration time: 20 µs
Tryptic digest of gel lanes for analysis with MALDI-TOF
Note:
- Make sure to work under a fume hood.
- Do not work with protective gloves to prevent contamination of your sample with platicizers.
Reaction tubes have to be cleaned with 60% (v/v) CH3CN, 0.1% (v/v) TFA. Afterwards the solution has to be removed completely followed by evaporation of the tubes under a fume hood. Alternatively microtiter plates from Greiner® (REF 650161) can be used without washing.
- Cut out the protein lanes of a Coomassie-stained SDS-PAGE using a clean scalpel. Gel parts are transferred to the washed reaction tubes/microtiter plate. If necessary cut the parts to smaller slices.
- Gel slices should be washed two times. Therefore add 200 µL 30% (v/v) acetonitrile in 0.1 M ammonium hydrogen carbonate each time and shake lightly for 10 minutes. Remove supernatant and discard to special waste.
- Dry gel slices at least 30 minutes in a Speedvac.
- Rehydrate gel slices in 15 µL Trypsin-solution followed by short centrifugation.
- Gel slices have to be incubated 30 minutes at room temperature, followed by incubation at 37 °C over night.
- Dry gel slices at least 30 minutes in a Speedvac.
- According to the size of the gel slice, add 5 – 20 µL 50% (v/v) ACN / 0,1% (v/v) TFA.
- Samples can be used for MALDI measurement or stored at -20 °C.
Trypsin-solution: 1 µL Trypsin + 14 µL 10 mM NH4HCO3
- Therefore solubilize lyophilized Trypsin in 200 µL of provided buffer and incubate for 15 minutes at 30 °C for activation. For further use it can be stored at -20 °C.
Preparation and Spotting for analysis of peptides on Bruker AnchorChips
- Spot 0,5 – 1 µL sample aliquot
- Add 1 µL HCCA matrix solution to the spotted sample aliquots. Pipet up and down approximately five times to obtain a sufficient mixing. Be careful not to contact the AnchorChip.
Note: Most of the sample solvent needs to be gone in order to achieve a sufficiently low water content. When the matrix solution is added to the previously spotted sample aliquot at a too high water content in the mixture, it will result in undesired crystallization of the matrix outside the anchor spot area.
- Dry the prepared spots at room temperature
- Spot external calibrants on the adjacent calibrant spot positions. Use the calibrant stock solution (Bruker’s “Peptide Calibration Standard II”, Part number #222570), add 125 µL of 0,1% TFA (v/v) in 30% ACN to the vial. Vortex and sonicate the vial.
- Mix the calibrant stock solution in a 1:200 ratio with HCCA matrix and deposit 1 µL of the mixture onto the calibrant spots.
Release of periplasmic protein fraction from E. coli by cold osmotic shock
- Modified protocol from Neu & Heppel, 1965.
- Centrifuge E. coli cell suspension for 5 min at 14000 g (4 °C) to collect the cells.
- Discard the entire supernatant.
- Resuspend the cells ice-cold cell fractionating buffer #1. The resulting volume should be 1/4 of the former suspension volume.
- Incubate for 20 min on ice. Ivert the suspension at regular intervals to counteract sedimentation.
- Centrifuge the cell suspension for 15 min at 14000 g (4 °C).
- Discard the entire supernatant.
- Resuspend the cells ice-cold cell fractionating buffer #2. The resulting volume should be 1/4 of the former suspension volume.
- Incubate for 10 min on ice under regular invertion.
- Centrifuge the cell suspension for 15 min at 14000 g (4 °C).
- Save the supernatant, which contains the periplasmatic proteins.
- If the periplasmatic protein fraction is turbid, re-centrifuge and filter it through a 0.2 µm filter.
References
Hansmeier N, Bartels FW, Ros R, Anselmetti D, Tauch A, Pühler A, Kalinowski J (2004) Classification of hyper-variable Corynebacterium glutamicum surface-layer proteins by sequence analyses and atomic force microscopy [http://www.sciencedirect.com/science/article/pii/S016816560400241X J Biotechnol. 26;112(1-2):177-93.]
Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM (2007) S-layers as a tool kit for nanobiotechnological applications, [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full FEMS Microbiol Lett 267(2):131-144].