Difference between revisions of "Part:BBa K525311"
| (12 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K525311 short</partinfo> | <partinfo>BBa_K525311 short</partinfo> | ||
| + | |||
| + | [[Image:Bielefeld-Germany2011-S-Layer-Geometrien.jpg|300px|thumb|right]] | ||
Fusion protein of S-layer SgsE and firefly luciferase. | Fusion protein of S-layer SgsE and firefly luciferase. | ||
| Line 22: | Line 24: | ||
|rowspan="3"|Expression (''E. coli'') | |rowspan="3"|Expression (''E. coli'') | ||
|Localisation | |Localisation | ||
| − | | | + | |Some soluble, mostly inclusion bodies |
|- | |- | ||
|Compatibility | |Compatibility | ||
| Line 43: | Line 45: | ||
|Immobilization time | |Immobilization time | ||
|4 h | |4 h | ||
| + | |- | ||
| + | |Materials binding <partinfo>K525311</partinfo> | ||
| + | |Silicon dioxide, polyethylene sulfone | ||
|- | |- | ||
|} | |} | ||
| Line 57: | Line 62: | ||
<partinfo>BBa_K525311 parameters</partinfo> | <partinfo>BBa_K525311 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ===Expression in ''E. coli''=== | ||
| + | The SgsE gene under the control of a T7 / lac promoter (<partinfo>K525303</partinfo>) was fused to firefly luciferase (<partinfo>K525999</partinfo>) using Freiburg BioBrick assembly for characterization experiments. | ||
| + | |||
| + | The SgsE|luciferase fusion protein was overexpressed in ''E. coli'' KRX after induction of T7 polymerase with 0.2 % L-rhamnose and induction of lac operator with 1 mM IPTG. | ||
| + | |||
| + | The following cultivation was carried out in a [http://www.gmi-inc.com/BioEngineering-KLF-Small-Laboratory-Fermenter.html#product_desc Bioengineering KLF] bioreactor with Bioengineering DCU and software. A sequencer which automatically pumped an inducer solution after 4 h cultivation time to start protein expression was implemented. Other parameters were: | ||
| + | |||
| + | * Medium: [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_medium HSG medium] with 20 mg L<sup>-1</sup> chloramphenicol | ||
| + | * Culture volume: 2.5 L | ||
| + | * Starting OD<sub>600</sub>: 0.4 | ||
| + | * DO: 60 % airsaturation (controlled with stirrer cascade starting with 200 rpm) | ||
| + | * pH: 7.0 (controlled with 20 % phosphoric acid and 2 M NaOH) | ||
| + | * Antifoam: BASF pluronic PE-8100 | ||
| + | * Induction solution: 0.2 % L-rhamnose and 1 mM IPTG | ||
| + | |||
| + | |||
| + | The following figure shows the expression of the SgsE | luciferase S-layer fusion protein <partinfo>K525311</partinfo> in ''E. coli'' KRX in HSG medium with autoinduction sequencer as described above. Optical density, activity of the fused luciferase, dissolved oxygen and agitator speed are plotted against the cultivation time. | ||
| + | |||
| + | [[Image:IGEM-Bielefeld2011-Cultivation311.jpg|750px|thumb|center|'''Fig. 1: Bioreactor cultivation of ''E. coli'' KRX expressing the fusion protein SgsE :: luciferase <partinfo>K525311</partinfo> under the control of a T7 / lac promoter. Induction with 1 mM IPTG and 0.2 % L-rhamnose after 4 h controlled by sequencer. Cultivation in [http://www.gmi-inc.com/BioEngineering-KLF-Small-Laboratory-Fermenter.html#product_desc Bioengineering KLF] bioreactor with 2.5 L [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_medium HSG medium] with 20 mg L<sup>-1</sup> chloramphenicol starting with OD<sub>600</sub> = 0.4. Dissolved oxygen was regulated to 60 % airsaturation (controlled with stirrer cascade starting with 200 rpm) and the pH to 7.0 (controlled with 20 % phosphoric acid and 2 M NaOH). The dissolved oxygen sensor had to be recalibrated after about 140 min. ''']] | ||
| + | |||
| + | |||
| + | ===Purification=== | ||
| + | After the analysis of cultivations with expression of SgsE | luciferase fusion proteins, different cell fractions were analyzed. It could be seen that the proteins form inclusion bodies in ''E. coli'' but that there are some soluble proteins left. This has the advantage that the proteins carrying an enzyme as fusion proteins do not have to be treated with denaturating agents like urea which destroys the enzyme (data not shown). | ||
| + | |||
| + | To capture the protein from the cell lysate an ion exchange chromatography (IEX) was carried out (binding with pH 7.0, 25 mM NaCl, quaternary amine beads, elution with 100 mM NaCl). A lot of protein was found in the flow-through. When concentrating and rebuffering the proteins with PES (polyethylene sulfone) membranes a lot of protein was lost. The S-layer proteins stuck to the membrane. Some could be removed again from the membrane after cutting out the filter and incubate it in ddH<sub>2</sub>O over-night. This problem has to be kept in mind when using this S-layer. | ||
| + | |||
| + | The results of the purification approach are shown in Fig. 2: | ||
| + | |||
| + | [[Image:IGEM-Bielefeld2011-311purification.png|500px|thumb|center|'''Fig. 2: Luminescence corrected with the volume of the collected fraction plotted against the fraction during purification of <partinfo>K525311</partinfo>. Abbreviations: s.n. = supernatant, F = filtrate, R = retentate, FT = flow through, E10 = elution with 100 mM NaCl.''']] | ||
| + | |||
| + | The purification strategy has to be improved. The inclusion bodies cannot be purified because urea damages the luciferase irreversible (data not shown). The loss due to adsorption of the SgsE | luciferase fusion protein to PES membranes could be avoided by using different membranes. The binding conditions of the IEX have to be improved as well. Anyway, the idea behind this purification strategy could be a starting point for a better strategy. Possibilities for improvement are: | ||
| + | * Different membranes for ultra- / diafiltration | ||
| + | * Other binding conditions for the IEX capturing step (higher pH) | ||
| + | * Hydrophobic interaction chromatography as purification step after IEX (works in general, data not shown) | ||
| + | * Size exclusion chromatography (SEC) for polishing | ||
| + | |||
| + | |||
| + | |||
| + | ===Stability=== | ||
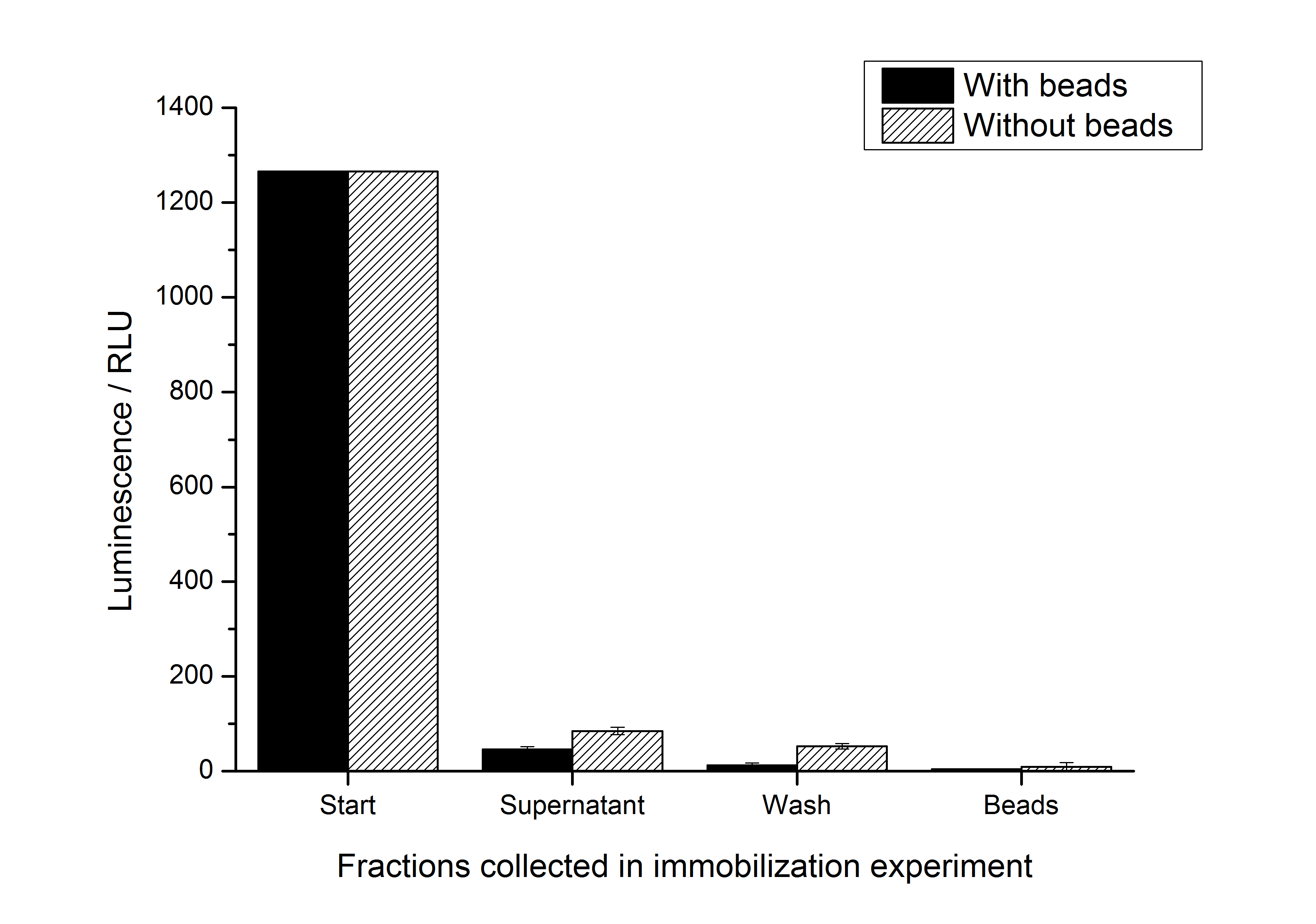
| + | To test whether the S-layer protein enhances the half-life of the firefly luciferase, immobilization experiments were carried out. After the IEX described above, the elution fraction was immobilized on silicon dioxide beads or just diluted with HBSS buffer which is used for the immobilization / recrystallization of SgsE S-layer proteins. The immobilization is carried out at room temperature for 4 h. It could be seen that the luciferase activity nearly expired during this time (in the positive and the negative control). So the S-layer SgsE could not stabilize the luciferase at room temperature. The results of this experiment is shown in the figure below: | ||
| + | |||
| + | [[Image:IGEM-Bielefeld2011-311immobilization.png|500px|thumb|center|'''Fig. 3: Results of immobilization experiments with <partinfo>K525311</partinfo>. The stability of the luciferase activity at room temperature is decreasing quickly.''']] | ||
Latest revision as of 23:19, 28 October 2011
Fusion Protein of S-Layer SgsE and Firefly-Luciferase
Fusion protein of S-layer SgsE and firefly luciferase.
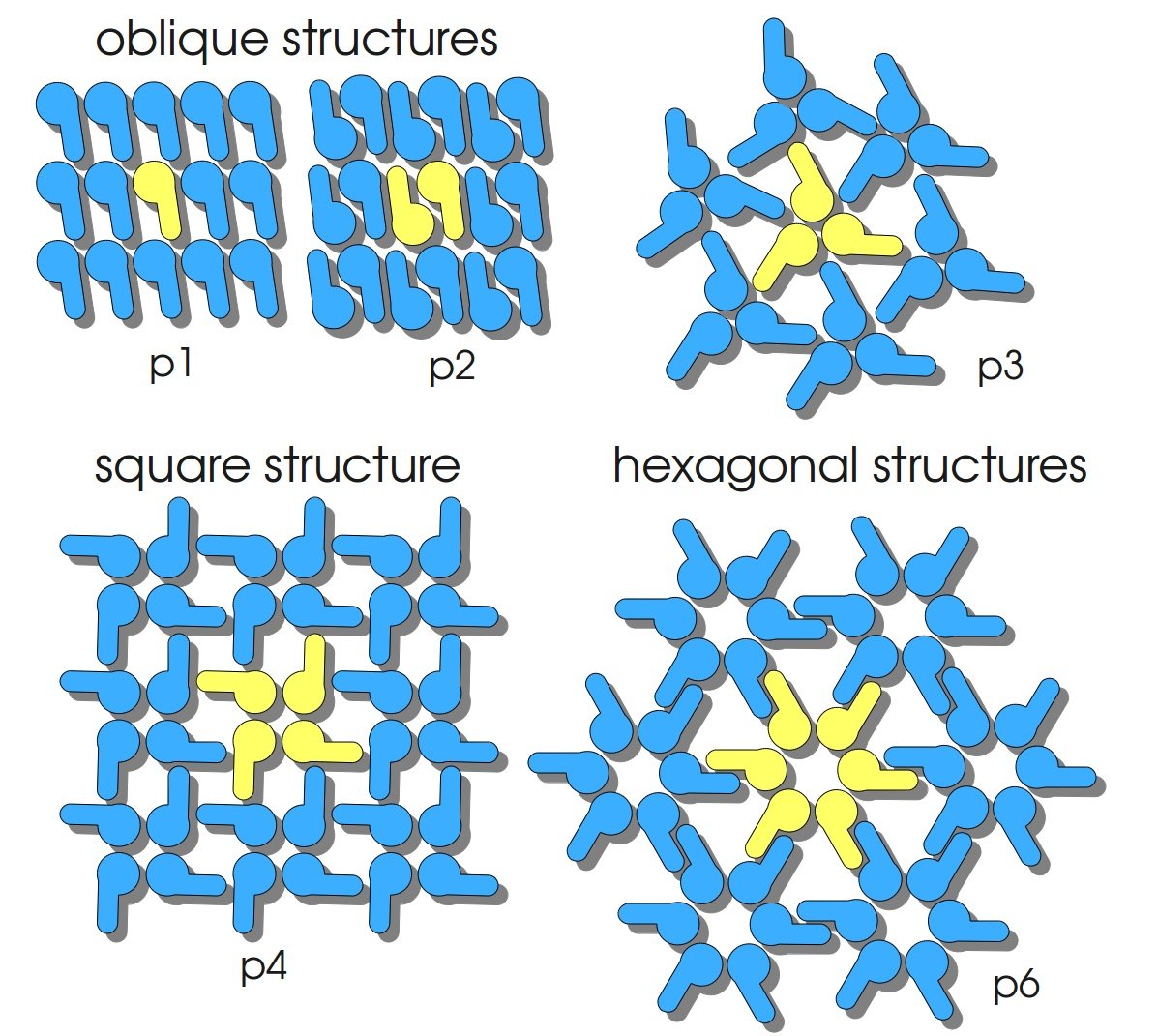
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
This S-layer fusion protein is used to characterize purification methods and immobilization behaviour of enzymes fused to an S-layer.
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Localisation | Some soluble, mostly inclusion bodies |
| Compatibility | E. coli KRX and BL21(DE3) | |
| Induction of expression | expression of T7 polymerase + IPTG or lactose | |
| Purification | Molecular weight | 143.8 kDa |
| Theoretical pI | 6.02 | |
| Reporter | Luminescence | |
| Immobilization behaviour | Immobilization time | 4 h |
| Materials binding BBa_K525311 | Silicon dioxide, polyethylene sulfone |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 167
Illegal BglII site found at 1022 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 76
Illegal AgeI site found at 4054 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1657
Illegal SapI.rc site found at 3211
Expression in E. coli
The SgsE gene under the control of a T7 / lac promoter (BBa_K525303) was fused to firefly luciferase (BBa_K525999) using Freiburg BioBrick assembly for characterization experiments.
The SgsE|luciferase fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase with 0.2 % L-rhamnose and induction of lac operator with 1 mM IPTG.
The following cultivation was carried out in a [http://www.gmi-inc.com/BioEngineering-KLF-Small-Laboratory-Fermenter.html#product_desc Bioengineering KLF] bioreactor with Bioengineering DCU and software. A sequencer which automatically pumped an inducer solution after 4 h cultivation time to start protein expression was implemented. Other parameters were:
- Medium: [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_medium HSG medium] with 20 mg L-1 chloramphenicol
- Culture volume: 2.5 L
- Starting OD600: 0.4
- DO: 60 % airsaturation (controlled with stirrer cascade starting with 200 rpm)
- pH: 7.0 (controlled with 20 % phosphoric acid and 2 M NaOH)
- Antifoam: BASF pluronic PE-8100
- Induction solution: 0.2 % L-rhamnose and 1 mM IPTG
The following figure shows the expression of the SgsE | luciferase S-layer fusion protein BBa_K525311 in E. coli KRX in HSG medium with autoinduction sequencer as described above. Optical density, activity of the fused luciferase, dissolved oxygen and agitator speed are plotted against the cultivation time.
Purification
After the analysis of cultivations with expression of SgsE | luciferase fusion proteins, different cell fractions were analyzed. It could be seen that the proteins form inclusion bodies in E. coli but that there are some soluble proteins left. This has the advantage that the proteins carrying an enzyme as fusion proteins do not have to be treated with denaturating agents like urea which destroys the enzyme (data not shown).
To capture the protein from the cell lysate an ion exchange chromatography (IEX) was carried out (binding with pH 7.0, 25 mM NaCl, quaternary amine beads, elution with 100 mM NaCl). A lot of protein was found in the flow-through. When concentrating and rebuffering the proteins with PES (polyethylene sulfone) membranes a lot of protein was lost. The S-layer proteins stuck to the membrane. Some could be removed again from the membrane after cutting out the filter and incubate it in ddH2O over-night. This problem has to be kept in mind when using this S-layer.
The results of the purification approach are shown in Fig. 2:
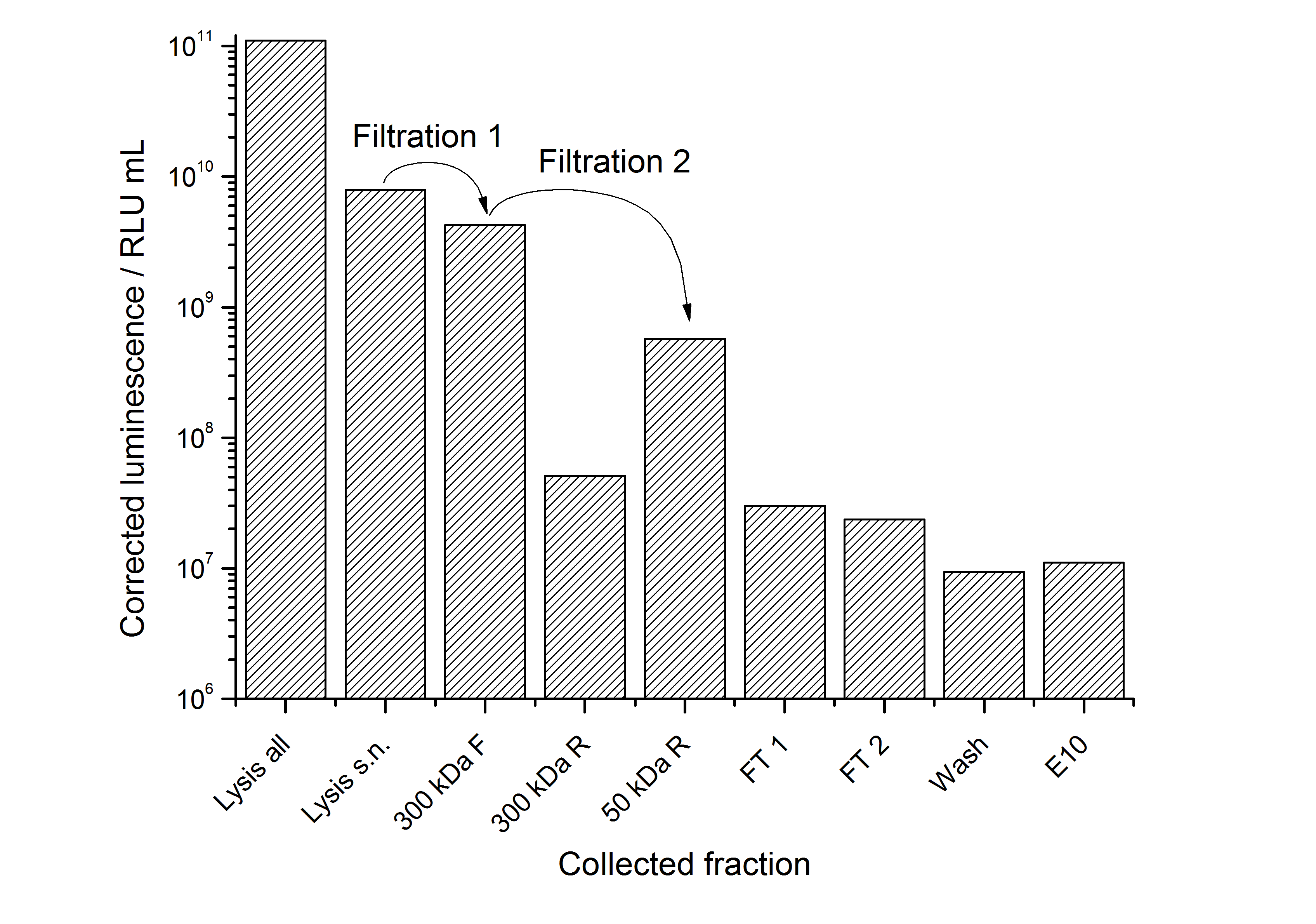
The purification strategy has to be improved. The inclusion bodies cannot be purified because urea damages the luciferase irreversible (data not shown). The loss due to adsorption of the SgsE | luciferase fusion protein to PES membranes could be avoided by using different membranes. The binding conditions of the IEX have to be improved as well. Anyway, the idea behind this purification strategy could be a starting point for a better strategy. Possibilities for improvement are:
- Different membranes for ultra- / diafiltration
- Other binding conditions for the IEX capturing step (higher pH)
- Hydrophobic interaction chromatography as purification step after IEX (works in general, data not shown)
- Size exclusion chromatography (SEC) for polishing
Stability
To test whether the S-layer protein enhances the half-life of the firefly luciferase, immobilization experiments were carried out. After the IEX described above, the elution fraction was immobilized on silicon dioxide beads or just diluted with HBSS buffer which is used for the immobilization / recrystallization of SgsE S-layer proteins. The immobilization is carried out at room temperature for 4 h. It could be seen that the luciferase activity nearly expired during this time (in the positive and the negative control). So the S-layer SgsE could not stabilize the luciferase at room temperature. The results of this experiment is shown in the figure below: