Difference between revisions of "Part:BBa K274210"
| Line 20: | Line 20: | ||
Over night (ON) cultures were grown from 4 colonies, until the following OD’s were obtained: | Over night (ON) cultures were grown from 4 colonies, until the following OD’s were obtained: | ||
| − | Top 10 - no insert | + | Top 10 - no insert OD = 0,008 (100 x diluted)<br> |
Top 10 - with K274210 insert OD = 0,011 (100 x diluted)<br> | Top 10 - with K274210 insert OD = 0,011 (100 x diluted)<br> | ||
MG1655 - no insert OD = 0,020 (100 x diluted)<br> | MG1655 - no insert OD = 0,020 (100 x diluted)<br> | ||
Revision as of 08:50, 31 August 2010
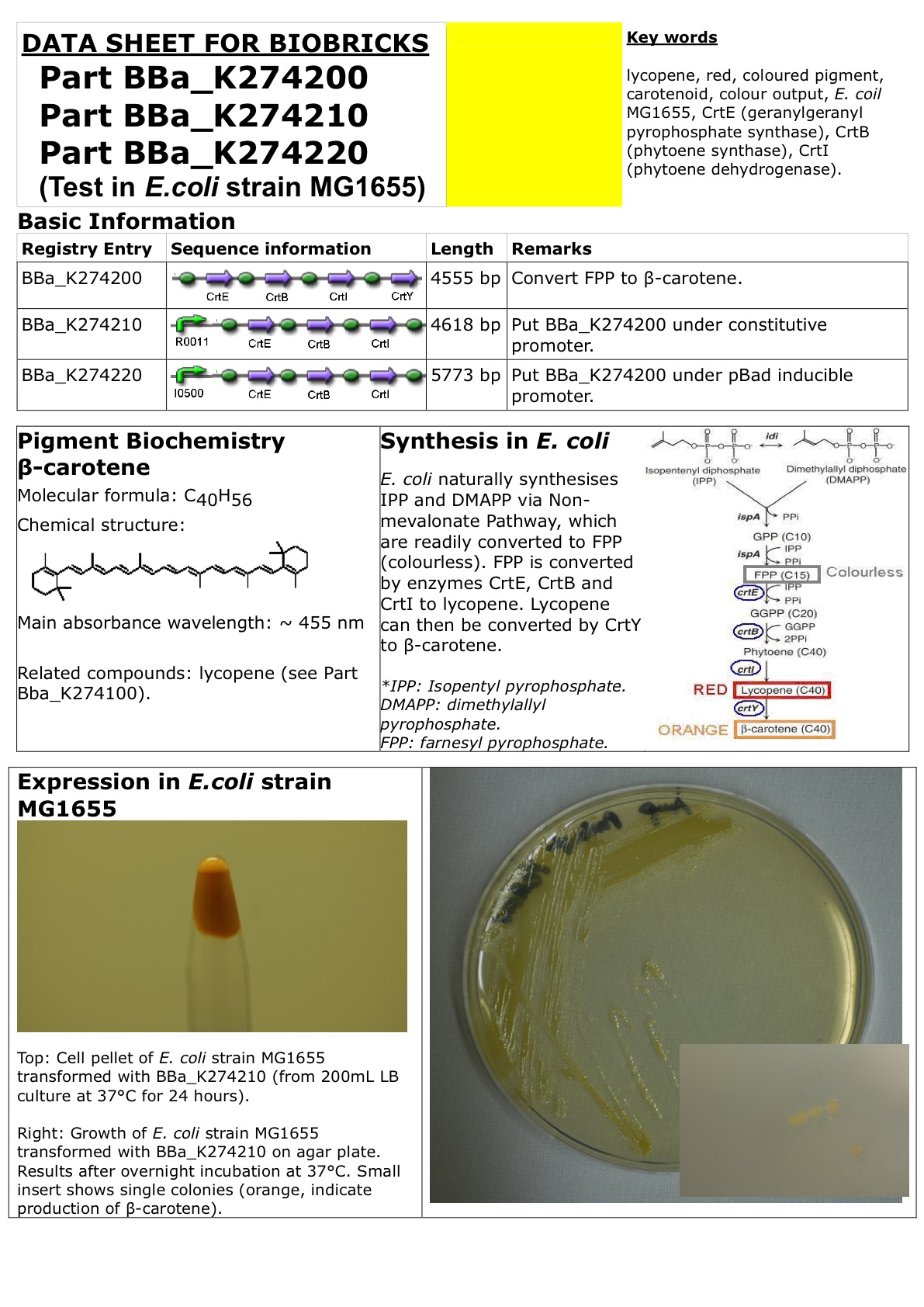
CrtEBIY under constitutive promoter
This Biobrick is created by putting enzyme cassette CrtEBIY (with individual rbs) of Part BBa_K274200 under constitutive promoter R0011.
Enzyme cassette CrtEBIY (with individual rbs) of Part BBa_K274200 converts colourless farnesyl pyrophosphate to orange beta-carotene (via intermediates geranylgeranyl pyroiphosphate, phytoene and lycopene).
Amount of beta-carotene produced can be measured by photospectrometer with absorbance at 455nm (beta-carotene extraction using acetone).
Datasheet for Part BBa_K274200, Part BBa_274210 and Part BBa_274220 in E. coli strain MG1655. You may also wish to refer to the "Experience" page.
For PDF version of this Datasheet: File:Carotene.pdf
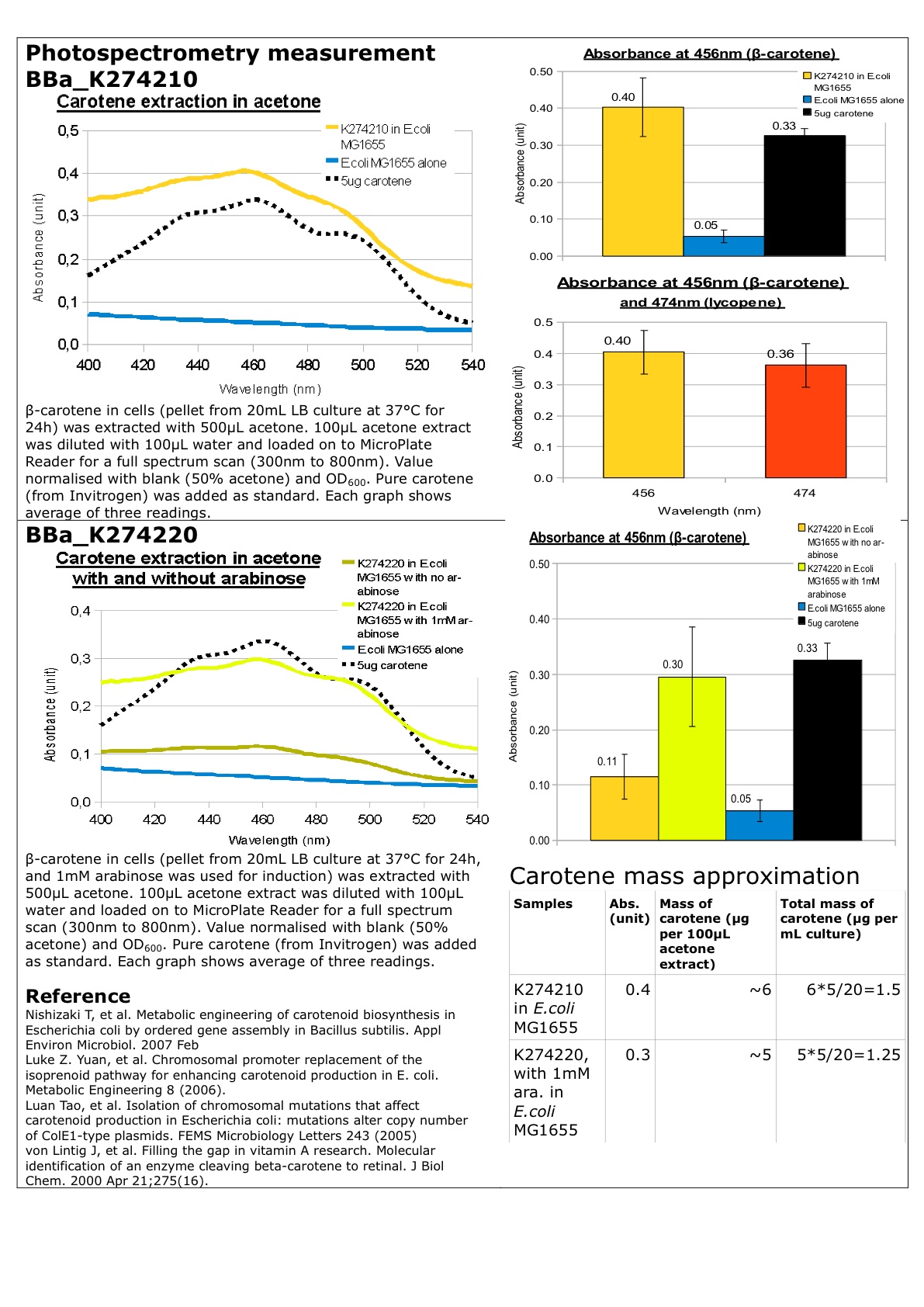
Further characterization of the BioBrick K274210 has been performed in Top10 and MG1655 E. coli. The experimental data have shown somewhat similar results for Top10 and MG1655 E. coli.
The experiment was performed as follows:
Over night (ON) cultures were grown from 4 colonies, until the following OD’s were obtained:
Top 10 - no insert OD = 0,008 (100 x diluted)
Top 10 - with K274210 insert OD = 0,011 (100 x diluted)
MG1655 - no insert OD = 0,020 (100 x diluted)
Mg1655 - with K274210 insert OD = 0,017 (100 x diluted)
ON cultures were grown in 110 ml LB media. Colonies with K274210 insert were grown in LB media containing ampicillin. All ON cultures were grown for 20 hours at 37 °C. After 16 hours, 10 ml of the ON cultures were transferred into 110 ml LB media and grown for 4 hours to reach the exponential phase, where the following OD’s were obtained:
Top 10 - no insert OD = 0,049 (100 x diluted)
Top 10 - with K274210 insert OD = 0,044 (100 x diluted)
MG1655 - no insert OD = 0,007 (100 x diluted)
Mg1655 - with K274210 insert OD = 0,009 (100 x diluted)
Cultures with K274210 insert were grown in media containing ampicillin.
100 mL cell culture were centrifuged for 5 min at 14000 RPM. The supernatant was discarded and cells were resuspended in 5 mL acetone (99,9%), except the Top 10 E. coli with the K274210 insert, which was resuspended in 10 mL acetone (source of error). The resuspended cells were sonicated for 5 min. Samples were spun down, the supernatant was transferred to new tubes, and cell debris was discarded. A standard curve was made from pure beta-carotene.
The samples at the OD’s seen above as well as solutions of pure beta-carotene with known concentrations were measured at a fixed wavelength of 456 nm. Known concentrations and their absorbances:
| Concentration | Absorbance |
| 1 mM | 4,000 |
| 100 µM | 2,260 |
| 50 µM | 4,000 |
| 25 µM | 0,155 |
| 10 µM | 0,893 |
| 5 µM | 0,440 |
| 1 µM | 0,075 |
| 100 nM | 0,015 |
| 10 nM | 0,038 |
| 1 nM | 0,005 |
| 100 pM | 0,024 |
The samples and their absorbances:
| Top 10 cells (Absorbance) | MG1655 E. coli mutant (Absorbance) | |
| Stationary phase control | 0,034 | 0,024 |
| Stationary phase with K274210 biobrick insert | 0,319 | 1,549 |
| Expotential phase control | 0,020 | 0,024 |
| Expotential phase with K274210 biobrick insert | 0,034 | 0,033 |
UV-VIS absorbance spectra of the known solutions were obtained, as well as spectra of the samples at the ODs shown above. The spectra of Top 10 and MG1655 in the stationary phase as well as selected spectra of known solutions are shown below:

These results were obtained from one experiment, which will be replicated later for more precision.
Reference
Hal Alper, et al. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nature Biotechnology 23 (2005).
Nishizaki T, et al. Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl Environ Microbiol. 2007 Feb
Luke Z. Yuan, et al. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metabolic Engineering 8 (2006).
Luan Tao, et al. Isolation of chromosomal mutations that affect carotenoid production in Escherichia coli: mutations alter copy number of ColE1-type plasmids. FEMS Microbiology Letters 243 (2005)
von Lintig J, et al. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000 Apr 21;275(16).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 2037
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1573
Illegal NgoMIV site found at 1703
Illegal AgeI site found at 788 - 1000COMPATIBLE WITH RFC[1000]