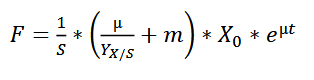Difference between revisions of "Part:BBa K4247005"
(→Improvements by Imperial-College 2024) |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 456: | Line 456: | ||
'''Conclusion - '''It is very evident that switching the His-tag from the C-terminus to N-terminus increased the protein production of the Minispidroin protein substantially, by nearly 8 times. | '''Conclusion - '''It is very evident that switching the His-tag from the C-terminus to N-terminus increased the protein production of the Minispidroin protein substantially, by nearly 8 times. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-- ----------------------------------End of the Original Documentation ----------------------------------- --> | ||
| + | |||
| + | =<b>Improvementes and Uses: </b>= | ||
| + | |||
| + | ==Improvements by Imperial-College 2024== | ||
| + | <html> | ||
| + | <img src="https://static.igem.wiki/teams/5416/logo-name-t.png" alt="Description of image" width="200" /> | ||
| + | <figcaption><strong>Imperial-College 2024</strong></figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | This Part has been improved by Team Imperial-College 2024, where an artifical organelle inspired by the spider silk micelle formation is designed as a rubber producing nanoreactor, detailed in their <b>[[Part:BBa_K5416070|favourite basic parts page]]</b>. | ||
| + | <br> | ||
| + | <br> | ||
| + | Their designed basic part now consists of three major domains, the [[Part:BBa_K5416000|HRT2trunc]]; the NT domain which is this part; and an artifical hydrophobic surfactant protein (C33Leu) which mediates the formation of hydrophobic compartments. | ||
| + | |||
| + | |||
| + | <html> | ||
| + | <div align="center"> | ||
| + | <img src="https://static.igem.wiki/teams/5416/parts/hrt2-nt-asip/fig2-background.png" width="400px"> | ||
| + | <p><small>Fig 1: the proposed micelle formation mechanism of this part. Created with Biorender.</small></p> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | ===Highlights of Results=== | ||
| + | We have investigated the formation of our artificial micelle using this part. This has been performed using a BODIPY staining technique, which the BODIPY molecule stains specifically with intracellular hydrophobic pockets via interactions with aliphatic polymers such as triglycerides and polyisoprene (rubber). | ||
| + | |||
| + | <html><div align ="center"> | ||
| + | <img src="https://static.igem.wiki/teams/5416/parts/hrt2-nt-asip/fig7-bodipy-reading.png" width="300px"> | ||
| + | <p><small>Fig.2: The fluorescent reading with excitation at 485nm and emission at 520nm for the cells stained with BODIPY. pET-28a(+) (pET) transformed BL21 induced with both 0mM and 1mM IPTG at OD600 = 0.5 serves as the global negative control (grey). The transformants of pET28a_HRT2trunc-NT-ASIP (ASIP) is induced with same IPTG conditions, where all cells are then cultured at 30oC for 16hr. The experiment is carried with 5 repeats of each sample, where the max and min from each group are represented by the error bars. The mean is shown with a cross, and the medium is identified as with the line inside the box. The box represents the interquartile range of the data distribution.</small></p> | ||
| + | </div></html> | ||
| + | |||
| + | After rinsing the strained cells 3 times with PBSG (5% glycerol in PBS) to remove excess BODIPY molecule inside the cell membrane, the fluorescence of each group of cells were measured <sup>[7]</sup>. It was then observed that the HRT2trunc-NT-ASIP expressing cell (ASIP 1mM IPTG) produces significantly more fluorescence comparing to the non-induced transformant and negative controls. A one-tailed student t test was hence conducted to further compare the induced and non-induced groups, where solid statistical evidence was given with p<0.001 to further justify this observation. The average fluorescence of the induced cell is over <b>158% higher</b> than the cells with no inducer added. | ||
| + | |||
| + | To further evident that our HRT2trunc-NT-ASIP is forming an artificial organelle that holds hydrophobic rubber molecule. We then imaged the BODIPY stained cells under a confocal microscope to identify the location of the stains inside the bacteria. | ||
| + | |||
| + | <html><div align ="center"> | ||
| + | <img src="https://static.igem.wiki/teams/5416/parts/hrt2-nt-asip/fig8-asipconfocal.png" width="400px"> | ||
| + | <p><small>Fig.3 (Left) Positive group, HRT2trun-NT-ASIP with IPTG induced; (Right) Negative group, without being IPTG induced. Notice the yellow signal in the central of individual cells of IPTG induced sample. The samples were observed at 100x magnification.</small></p> | ||
| + | </div></html> | ||
| + | |||
| + | The confocal microscopy images have revealed significantly higher and more nucleated fluorescence signal inside the HRT2trun-NT-ASIP expressing E. coli. Whereby the Z-stack images which scanned through the E. coli cell have evidenced that the signal cluster is present inside the cell rather attaching to the cell membrane. This visually evidenced that the ASIP is mediating the formation of artificial hydrophobic chamber inside the E. coli after translated. Since BODIPY selectively dyes hydrocarbons, it is then highly evidence that these organelles are very likely to carry natural rubber. | ||
| + | |||
| + | The part was also characterized for its ability to produce natural rubber. The E. coli expressing this part is cultured in large quantity (100ml) and induced overnight with 1mM IPTG at 30C with a pET negative control after reaching OD600 = 0.5. The cells were lysed through the chemical method (detailed at Imperial_college_2024 wiki, contribution page), where rubber is extracted using cyclohexane. The absorbance at the UV spectrum was then measured for the solutions. | ||
| + | |||
| + | <html><div align ="center"> | ||
| + | <img src="https://static.igem.wiki/teams/5416/parts/hrt2-nt-asip/fig9-asiprubberextract.png" width="300px"> | ||
| + | <p><small>Fig.4 (A): UV-vis absorbance curve of rubber extracted from the overnight cultures with cyclohexane. pET serves as a negative control. (B): comparing the absorbance value acquired at 210nm of different samples that contain natural rubber (cis-1,4-polyisoprene), ASIP is the HRT2trunc-NT-ASIP parts addressed in this registry.</small></p> | ||
| + | </div></html> | ||
| + | |||
| + | The curve for ASIP is observed to be overall higher than the control group. Indicating a higher amount of polyisoprene being extracted with cyclohexane. The extract from cells expressing this part has also been compared with wild-type (pET28a) E. coli with known amounts of polyisoprene added. Through comparing the absorbance at 210nm a theoretical yield is thus determined to be around 0.1 to 0.5mg per 100ml of culture.<br><br> | ||
| + | <html> | ||
| + | <p><center><strong>----- END-OF-DOCUMNETATION IMPERIAL_COLLEGE2024 -----</strong></center></P> | ||
| + | </html> | ||
Latest revision as of 15:31, 1 October 2024
Contents
- 1 Minispidroin_NT_N-6His
- 2 Usage and Biology
- 3 Characterization of Minispidroin_NT-2rep-CT_N-6His
- 3.1 Optimization of inducer concentration
- 3.2 Optimization of temperature after induction
- 3.3 Optimization of lysis buffer
- 3.4 Heat purification
- 3.5 pH purification
- 3.6 Optimization of media
- 3.7 Protein purification by IMAC
- 3.8 A quicker approach to protein purification by IMAC
- 3.9 Stripping the Ni-NTA columns with urea
- 3.10 Large scale production
- 3.11 Spinning
- 3.12 Protein yields
- 3.13 Comparison of protein production with respect to His-tag location
- 4 Characterization of Minispidroin_NT-4rep-CT_N-6His
- 5 Improvementes and Uses:
Minispidroin_NT_N-6His
This part codes for a 6x His-tag followed by the N-terminal domain of minispidroin, a highly soluble spider silk protein. This part, together with BBa_K4247001 and BBa_K4247002 gives the full sequence of the minispidroin protein with a 6x His-tag in the N-terminus.
This part is one of a collection of compatible minispidroin parts: BBa_K4247000 (Minispidroin_NT), BBa_K4247001 (Minispidroin_2rep), BBa_K4247002 (Minispidroin_CT), BBa_K4247004 (Minispidroin_NT-2rep-CT), BBa_K4247005 (Minispidroin_NT_N-6His), BBa_K4247007 (Minispidroin_NT-2rep-CT_N-6His), BBa_K4247010 (Minispidroin_NT-2rep-CT-SnoopTag_N-6His), BBa_K4247011 (Minispidroin_NT-4rep-CT), BBa_K4247012 (Minispidroin_NT-4rep-CT_N-6His), BBa_K4247013 (Minispidroin_NT-4rep-CT-SnoopTag_N-6His).
Usage and Biology
Dragline silk produced by spiders is one of the strongest natural materials to exist and it is mainly made up of structural proteins called spidroins. These spidroins consist of non-repetitive N-terminal and C-terminal domains and a repetitive central part consisting of tandem repeats of a certain amino acid sequence. These sequences are rich in alanine and glycine to form the crystalline and amorphous parts of the fibre respectively.
There are many research articles whose authors could successfully produce recombinant spider silk proteins and spin them into fibres by mimicking the conditions of the spider’s silk gland where the fibers are formed naturally. But a major drawback in many of these recombinant spidroins was their low solubility. It has been found that the N-terminus of the spidroin is highly soluble at neutral pH which contributes to the solubility of the protein.
In the spider's silk gland, before spinning, the spidroins remain in a highly concentrated and soluble state. Then, this highly concentrated spidroin solution called spinning dope is subject to a gradual drop in pH from 7.6 to 5.7 along the gland which triggers the formation of the fiber. This drop in pH triggers the N-terminus to be more stable and form large network-like structures whereas the C-terminus becomes more unstable to drive spontaneous fibre formation by forming the beta-sheet fibrils which form the core of the fiber. The N-terminal domain restricts the formation of silk fibers to a precise point in the silk duct, preventing silk proteins stored in the silk gland from agglutinating.
This clearly shows us that the solubility and pH sensitivity have a huge effect on the N- and C-terminus of the spidroin which thus affects the formation of fibers. It has been found that the N-terminus of MaSp1 (Major ampullate spidroin 1) from Euprosthenops australis, shows extremely high solubility and pH sensitivity whereas the C-terminus has low solubility and is inert to pH changes and vice versa for the MiSp (Minor ampullate spidroin) of Araneus ventricosus.
 Andersson et al., 2017 show how minispidroin can be spun into long fibers
Andersson et al., 2017 show how minispidroin can be spun into long fibers
Herein, part BBa_K4247005 contains the coding sequence for a 6x His-tag followed by the N-terminus of the minispidroin protein.
Characterization of Minispidroin_NT-2rep-CT_N-6His
Optimization of inducer concentration
Aim - To determine the concentration of inducer required for optimal protein expression.
Results - Cell cultures were grown ON at 37°C. Then, the next day, the cultures were diluted to an OD600 of 0.1 and induced with 0.1, 0.3, 0.5 and 1mM IPTG and grew ON. We can clearly see that around 30kDa, there is a darker band in the induced lanes compared to the uninduced lane, showing that the protein is expressed upon induction with IPTG. Further, among the induced lanes, protein expression is maximum when the cultures were induced with 0.3mM IPTG.
Further, a western blot was done on the above SDS-gel to confirm that the proteins we see are indeed the minispidroin proteins. Since the proteins were expressed with a 6x His-tag, we used mouse anti-hexa his primary antibodies and goat anti-mouse HRP-conjugated secondary antibodies for the western blot. Once again, it is clear that 0.3mM IPTG is the optimal inducer concentration.
Conclusion - So, it is clear that 0.3mM IPTG is the optimal concentration for protein expression. This is further backed up by the results of the minispidroin literature since the authors found 0.3mM IPTG to the optimal concentration too.
Optimization of temperature after induction
Aim - To determine the optimal temperature for growing the cells post-induction.
Results - The cultures were grown ON at 37°C. Then, the next day, the cultures were diluted to an OD600 of 0.1 and induced with 0.3mM IPTG and grew ON at 3 different temperatures - 20, 28 and 37°C. Similarly, uninduced controls were grown in identical conditions. The cells either have the 6x His-tag in the N-terminus or the C-terminus.
Conclusion - It can be seen that at all temperatures, the induced cells produce the proteins whereas the uninduced controls don’t and among the induced cultures, protein expression is highest when the cells are incubated at 28°C post-induction. Further, it is clear that more protein is expressed when the 6x His-tag is in N-terminus rather than the C-terminus of the protein.
Optimization of lysis buffer
Aim - To determine the best buffer for cell lysis that provides most of the protein in a soluble state.
Results - In order to lyse our cells by sonication, we used 2 different lysis buffers to decide which lysis buffer gave the most proteins in the soluble fraction. The recipes of the buffers are as follows,
Buffer 1: 50 mM NaH2PO4 + 500 mM sodium chloride + 10 mM imidazole + 0.5% Triton X-100 + 10% glycerol + 2 mM DTT (added fresh, right before use), pH 8.0
Buffer 2: 20mM Tris-Cl, pH 8.0
The cell cultures were centrifuged to obtain the cell pellets which were resuspended in the cell lysis buffer and then sonicated until a clear lysate was obtained. The lysate was centrifuged to obtain the insoluble and soluble fractions in the pellet and supernatant respectively. In the SDS-gel, we can clearly see that most of the protein is in the soluble fraction for both the buffers. Further, it is also clear that we obtain more of the protein in the soluble fraction with buffer 2 which is also the buffer used in the minispidroin literature.
Further, a western blot was done on the above SDS-gel to confirm that the proteins we see are indeed the minispidroin proteins using the above mentioned antibodies. Once again, it is clear that most of the protein is obtained in the soluble fraction with buffer 2.
Conclusion - From the SDS-gel and western blot, we can conclude that buffer 2 is better than buffer 1 since it provides most of the protein in the soluble fraction. This is further backed up by the results of the minispidroin literature since the authors used buffer 2 as well.
Heat purification
Aim - To determine if there is a temperature that would precipitate only the minispidroin proteins without the other E.coli proteins, to facilitate an easy purification method using heat treatment.
Results - The soluble fraction of the lysate was subject to different temperatures (37, 40, 45, 50, 60°C). Then, after heat treatment, the samples were centrifuged and the supernatants and pellets obtained after different temperatures were run on an SDS-gel. It is clear that minispidroin-NT_2rep_CT remains soluble upto 50°C since most of it is found in the supernatant and not the pellet. At 50 and 60°C, most of the protein is found in the pellet showing that it precipitates at temperatures above 50°C. However, a lot of other proteins also precipitate at those temperatures, so it is not possible to obtain the pure protein.
To test if higher temperatures would yield puree proteins, the lysate was subject to 70 and 80°C. It is clear that most of the protein precipitates at 70 and 80°C but like before, it is not very pure since a lot of other proteins are also precipitating.
Further, a western blot was done on the above SDS-gel to confirm that the proteins we see are indeed the minispidroin proteins using the above mentioned antibodies. Once again, it is clear that most of the protein precipitates at 70 and 80°C.
Conclusion - So, although most of the protein precipitates at temperatures above 50°C, heat treatment is not a suitable method for protein purification since there is no temperature that precipitates minispidroin without also precipitating other proteins.
pH purification
Aim - To determine if there is a certain pH at which minispidroin_NT-2rep-CT precipitates without the other E.coli proteins, to facilitate an easy purification method using changes in pH.
Results - The soluble fraction of the lysate was adjusted to different pH (5.5, 6, 6.5, 7, 7.5) and incubated for 1h at room temperature. Then, the samples were centrifuged and the supernatants and pellets obtained at different pH values were run on an SDS-gel. It is clear that minispidroin_NT-2rep-CT does not precipitate at any pH since the lanes with pellets do not have any bands around 30kDa.
Conclusion - Since there is no pH value at which the protein precipitates, the method of adjusting the pH cannot be used for purification.
Optimization of media
Aim - To determine which media, LB (Luria broth) or TB (Terrific broth) is better for protein expression.
Results - The cells were inoculated in either LB or TB media and grown ON at 37°C in identical conditions. The cultures were induced with 0.3mM IPTG and allowed to express the protein ON.
Conclusion - It is clear that more of the protein is expressed when the cells are grown in LB rather than TB.
Protein purification by IMAC
Aim - To purify the protein by IMAC (immobilized metal ion chromatography) using Ni-NTA resin.
Results - Mini columns were loaded with Ni-NTA resin and the soluble fraction of the lysate was added to the columns. Then, the columns were washed twice and eluted to obtain the purified protein. An SDS-gel was run on the different purification fractions and it is clear that most of the protein was lost in the flowthrough and washes and almost nothing was eluted. This shows that the proteins did not bind to the Ni-NTA column at all.
Lysis buffer - 20mM Tris-Cl, pH 8.0
Wash buffer - 20mM Tris-Cl, 5mM Imidazole, pH 8.0
Elution buffer - 20mM Tris-Cl, 200mM Imidazole, pH 8.0
Dialysis buffer - 20mM Tris-Cl, pH 8.0
Since most of the protein was lost in the flowthrough and washes in the previous attempt, the soluble fraction containing the proteins was allowed to incubate with the Ni-NTA resin under shaking conditions for 30-60 mins. This would provide sufficient time for the resin to bind the protein. Then, the columns were washed twice and eluted to obtain the purified protein. An SDS-gel was run on the different purification fractions and it is clear that most of the protein is obtained in the eluate and none of it is lost in the flowthrough or washes. Further, the purity of the obtained protein is high since there are no other bands.
Conclusion - So, with a sufficient incubation time (30-60mins) that allows the Ni-NTA resin to bind all the proteins, it is possible to elute the proteins with very high purity.
A quicker approach to protein purification by IMAC
Aim - To purify the protein by IMAC (immobilized metal ion chromatography) using Ni-NTA resin in a quicker way.
Results - Ni-NTA resin was added to the soluble fraction of the lysate and allowed to incubate for 1h under shaking conditions. Then, it was centrifuged to collect the resin with the proteins and the supernatant was collected as flowthrough. Then, the resin was washed twice and eluted 5 times.
Conclusion - It can be seen that most of the protein is collected in the first elution without much being lost in the flowthrough or the washes.
Stripping the Ni-NTA columns with urea
Aim - To wash the Ni-NTA columns with urea and strip them with EDTA after elution to determine if there was some aggregated protein in the resin or if there were any other metal binding proteins stuck to the column.
Results - After washes and elution, the column was washed with 8M urea. Any aggregated protein that is clumping the column would be solubilized in the urea and hence removed from the column. Then, the column was stripped using EDTA since EDTA can chelate metal ions and hence remove all the Ni and anything bound to it like metal binding proteins. We can see that while some of the protein is lost in the first wash, most of it is in the first elution. Further, a small amount of protein is also seen in the second elution and the urea wash and an even smaller amount after stripping the columns.
Conclusion - Although a small amount of protein is lost in the wash or stuck to the column, most of the protein is obtained in the first elution.
Large scale production
Aim - To carry out large scale protein production by fed-batch fermentation.
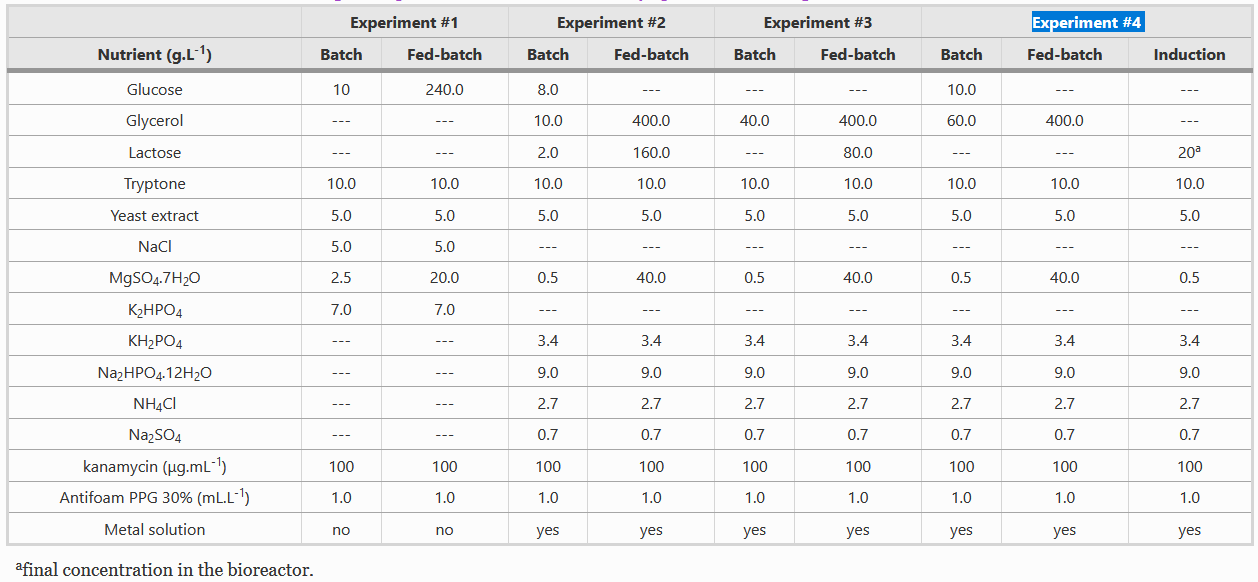
Composition of Batch and Fed-batch media
Instead of LB media, we used a complex growth medium that integrated a specific trace metal solution. The authors obtained this media from experiment #4 (see below) of another paper: A.J. da Silva, A.C.L. Horta, A.M. Velez, M.R.C. Iemma, C.R. Sargo, R.L.C. Giordano, M.T.M. Novo, R.C. Giordano, T.C. Zangirolami, Springerplus 2 (2013) 1–12.
For our bioreactor, we always started cultures with 1 L of batch media and set the system such that 500 mL of fed-batch media would enter the system at a specific rate and we used Silica antifoam.
The feeding rate should follow the formula:
wherein,
F - the rate of feeding (l h-1 )
S - concentration of the glycerol in the feed (g l-1 )
µ - specific growth rate (h-1)
YX/S - biomass yield on the substrate (g g-1 )
m - specific maintenance coefficient (g g-1 h -1 )
X - biomass concentration (g l-1 )
For the fermentation of this minispidroin protein, the authors set µ to 0.1, m to 0.025, and YX/S to 0.622.
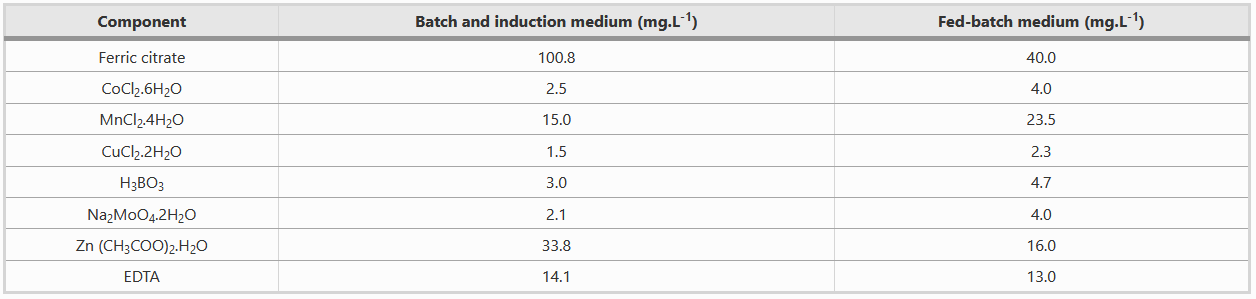
Composition of Metal solution
Both batch and fed-batch media have to be complemented with two different metal solutions, as shown in the table below.
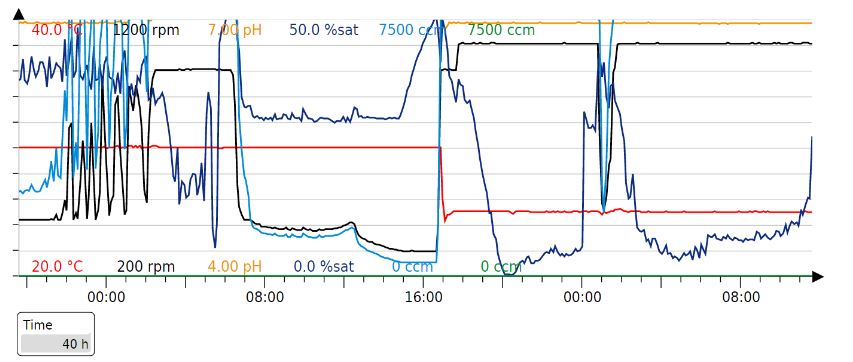
Parameters for the Fermentation cycle
Our fermentation strategy worked in every protein production cycle, but we had to slightly modify the timings.
Original parameters:
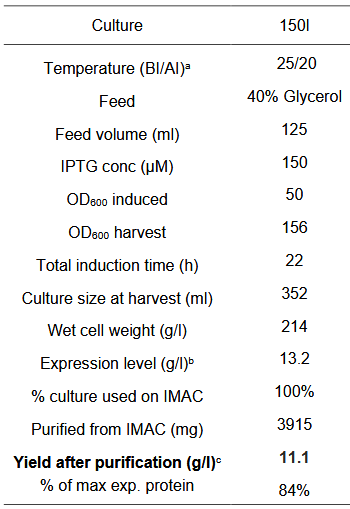
Our production cycle (40h):
Results - In order to obtain large amounts of the protein for spinning the fibre, the proteins were produced in large scale by cultivating the cells in a 1L fermenter. The cells were grown in 1L of batch media at 37°C to an OD600 of 77 which takes about 20h. Then, when the cells have consumed all the media, there is a spike in the oxygen saturation levels due to depletion of glucose. At this point, the cells are induced with 250uM IPTG and fed-batch (500ml) mode is started. Then, after 20h of induction, the cells are cultivated. An SDS-gel was done on cells before and after induction.
Then, the cells were lysed and the proteins were purified by IMAC using Ni-NTA resin. Although the purity is not very high as seen by a few other bands in the eluate lane, most of the protein is found in the eluate.
Conclusion - We can see that post-induction, the protein is produced by the cells in the fermenter. Further, purification by Ni-NTA chromatography was successful since a large band can be seen for the protein in the eluate showing that protein production has been increased.
Spinning
Aim - To spin the obtained purified proteins into a fibre.
Results - The purified proteins were dialyzed in a 10kDa cutoff membrane overnight against 20mM Tris-Cl, pH 8.0 to remove all the imidazole. Then, the dialyzed proteins were freeze dried to powder.
The powdered proteins were diluted to 30% w/v with 20mM Tris-Cl, pH 8.0. This concentrated spinning dope was used for spinning the fibre into a coagulation bath - 500 mM sodium acetate buffer, 200 mM NaCl, pH 5. It was extremely difficult to solubilise the powdered proteins and when we tried to spin the dope, the proteins just formed clumps in the coagulation bath as soon as they left the spinning setup.
Since it was extremely difficult to solubilize the powder at 30% w/v, we tried to solubilize the proteins at 15% w/v. This time, it was much easier to solubilize the protein in the buffer. However, when we tried to spin the fibre, the proteins formed clumps in the coagulation bath again and there was no fibre.
Since solubilizing the powdered proteins was difficult, we decided to concentrate the dialyzed proteins directly without freeze drying. We used Amicon 15 centrifugal filter units to concentrate the proteins into a highly concentrated, viscous spinning dope (~30% w/v). The spinning dope was spun into the same coagulation bath using the same spinning setup. As soon as the proteins hit the coagulation bath, they formed long fibres.
Conclusion - Hence, not only was it difficult to solubilize the freeze dried proteins but it was also not possible to spin the fibres from them whereas the proteins that were concentrated without freeze drying could be spun into long fibres.
Protein yields
Aim - To do a BCA assay after dialysis of the protein to quantify the final yield.
Results -
Minispidroin_NT-2rep-CT_N-6His was inoculated in LB, grown at 37°C, induced with 0.3mM IPTG and allowed to express the protein ON in a flask. The cells were lysed, the proteins purified by IMAC and dialysed ON. Then, a BCA assay was done to estimate the protein yields.
Conclusion - The yield of Minispidroin_NT-2rep-CT_N-6His was found to be 44.27 mg/L of culture.
Comparison of protein production with respect to His-tag location
Aim - To compare the protein production of the Minispidroin_NT-2rep_CT when the 6x His-tag was at the N-terminus or the C-terminus.
Results - Cells expressing minispidroin proteins with a His-tag in the N-terminus or C-terminus were grown to an OD600 of 0.1 and induced with 0.3mM IPTG to induce protein expression. Then, the cells were grown at 28°C post-induction ON. Then, the cells were lysed and the proteins were purified by IMAC. Further, the different purification fractions were run on an SDS-gel to compare the amount of proteins eluted.
Further, a BCA assay was done on the purified proteins to quantify the yields.
Conclusion - It is very evident that switching the His-tag from the C-terminus to N-terminus increased the protein production of the Minispidroin protein substantially, by nearly 8 times.
Characterization of Minispidroin_NT-4rep-CT_N-6His
Optimization of inducer concentration
Aim - To determine the concentration of inducer required for optimal protein expression, the weight of the desired protein is 40.3 KDa.
Results - Cell cultures were grown ON at 37°C. Then, the next day, the cultures were diluted to an OD600 of 0.1 and induced with 0.1, 0.3, 0.5 and 1mM IPTG and grew ON. We can clearly see that around 40kDa, there is a darker band in the induced lanes compared to the uninduced lane, showing that the protein is expressed upon induction with IPTG. Further, among the induced lanes, protein expression seems to be best in the range of 0.1-0.5 mM, but a western blot is needed for any clear conclusion.
A western blot was done on the above SDS-gel to confirm that the proteins we see are indeed the minispidroin proteins. Since the proteins were expressed with a 6x His-tag, we used mouse anti-hexa his primary antibodies and goat anti-mouse HRP-conjugated secondary antibodies for the western blot.
Conclusion - As observed in the western blot, IPTG concentration does not seem to impact particularly the expression of the protein. Considering that the shorter version of this minispidroin is expressed better at 0.3 mM, we decided to stick to this concentration for protein expression. This result aligns with the minispidroin literature since the authors found 0.3 mM IPTG to the optimal concentration but it also shows that IPTG concentration may not particularly impact protein expression of this protein.
Optimization of lysis buffer
Aim - To determine the best buffer for cell lysis that provides the protein in a soluble state.
Results - In order to lyse our cells by sonication, we used 2 different lysis buffers and then decided which lysis buffer gave the most proteins in the soluble fraction. The recipes of the buffers are as follows, Buffer 1: 50 mM NaH2PO4 + 500 mM sodium chloride + 10 mM imidazole + 0.5% Triton X-100 + 10% glycerol + 2 mM DTT (added right before use), pH 8.0 Buffer 2: 20mM Tris-Cl, pH 8.0
The cell cultures were centrifuged to obtain the cell pellets which were resuspended in the cell lysis buffer and then sonicated until a clear lysate was obtained. The lysate was centrifuged to obtain the insoluble and soluble fractions in the pellet and supernatant respectively. In the SDS-gel, minispidroin_NT-4rep_CT is visible in the buffer 2 supernatant.
Further, a western blot was done on the above SDS-gel to confirm that the proteins are indeed the minispidroin_NT-4rep-CT by using the above mentioned antibodies. Minispidroin_NT-4rep_CT is better obtained with buffer 2 since by using buffer 1 it seems to accumulate more in the pellet and would thus require a more difficult purification.
Conclusion - From the SDS-gel and western blot, we can conclude that buffer 2 is better than buffer 1 since it provides more protein in the soluble fraction. This confirms the results of the minispidroin literature since the authors used buffer 2.
Heat purification
Aim - To determine if there is a temperature that would precipitate only the minispidroin proteins without the other E.coli proteins, to facilitate an easy purification method using heat treatment.
Results - To test if higher temperatures would yield pure proteins or help separate minispidroin_Nt-4rep_CT from the native E. coli proteins, the lysate was subject to 70 and 80°C heat treatment for 20 minutes. It is clear that most of the protein precipitates at 70 and 80°C but it is not very pure since a lot of other proteins are also precipitating. We performed a western blot to confirm the finding. However, we decided to try lowering the heat treatment temperatures and check at what temperature the protein does precipitate. A note is that also in the controls, a low level of expression leads to the production of minimal amounts of the protein.
The soluble fraction of the lysate was subject to different temperatures (37, 40, 45, 50, 60°C) for 20 minutes. Then, after heat treatment, the samples were centrifuged and the supernatants and pellets obtained after different temperatures were run on an SDS-gel. It seems that minispidroin-NT_4rep_CT remains soluble up to 50°C. Again, a lot of other proteins also precipitate at those temperatures, so it is not possible to obtain the pure protein just by heat treatment.
Conclusion - So, although most of the protein precipitates at temperatures above 50°C, heat treatment is not a suitable method for protein purification since there is no temperature that precipitates minispidroin without also precipitating other proteins.
pH purification
Aim - To determine if there is a certain pH at which minispidroin_NT-4rep-CT precipitates without the other E.coli proteins, to facilitate an easy purification method using changes in pH.
Results - The soluble fraction of the lysate was adjusted to different pH (5.5, 6, 6.5, 7, 7.5) and incubated for 1h at room temperature. Then, the samples were centrifuged and the supernatants and pellets obtained at different pH values were run on an SDS-gel. It is clear that minispidroin_NT-4rep-CT does not precipitate at any pH since the lanes with pellets do not have any bands around 40kDa.
Conclusion - Since there is no pH value at which the protein precipitates, the method of adjusting the pH cannot be used for purification.
Protein purification by IMAC
Aim - To purify the protein by IMAC (immobilized metal ion chromatography) using Ni-NTA resin in a quicker way.
Results - Ni-NTA resin was added to the soluble fraction of the lysate and allowed to incubate for 1h under shaking conditions. Then, it was centrifuged to collect the resin with the proteins and the supernatant was collected as flowthrough. Then, the resin was washed twice and eluted 5 times.
Lysis buffer - 20mM Tris-Cl, pH 8.0
Wash buffer - 20mM Tris-Cl, 5mM Imidazole, pH 8.0
Elution buffer - 20mM Tris-Cl, 200mM Imidazole, pH 8.0
Dialysis buffer - 20mM Tris-Cl, pH 8.0
Conclusion - It can be seen that most of the protein is collected in the first elution without much of it being lost in the flowthrough or the washes.
Stripping the Ni-NTA columns with urea
Aim - To wash the Ni-NTA columns with urea and strip them with EDTA after elution to determine if there was some aggregated protein in the resin or if there were any other metal binding proteins stuck to the column.
Results - After washes and elution, the column was washed with 8M urea. Any aggregated protein that is clumping the column would be solubilized in the urea and hence removed from the column. Then, the column was stripped using EDTA since EDTA can chelate metal ions and hence remove all the Ni plus anything bound to, for example metal binding proteins. From the experiment it seems we did not lose protein in aggregates (urea lane) nor stuck to the Ni (EDTA). We can see that while some of the protein is lost in the first wash, most of it is in the first elution. Further, a small amount of protein is also seen in the second elution and the urea wash and an even smaller amount after stripping the columns.
Conclusion - Although a small amount of protein is lost in the wash or stuck to the column, most of the protein is obtained in the first elution. Moreover, dialysis worked as it can be seen in the following picture.
Protein yields
Aim - To do a BCA assay after dialysis of the protein to quantify the final yield.
Results -
Minispidroin_NT-4rep-CT_N-6His was inoculated in LB, grown at 37°C, induced with 0.3mM IPTG and allowed to express the protein ON in a flask. The cells were lysed, the proteins purified by IMAC and dialysed ON. Then, a BCA assay was done to estimate the protein yields.
Conclusion - The yield of Minispidroin_NT-4rep-CT_N-6His was found to be 48.39 mg/L of culture.
Comparison of protein production with respect to His-tag location
Aim - To compare the protein production of the Minispidroin_NT-4rep_CT when the 6x His-tag was at the N-terminus or the C-terminus.
Results - Cells expressing minispidroin proteins with a His-tag in the N-terminus or C-terminus were grown to an OD600 of 0.1 and induced with 0.3mM IPTG to induce protein expression. Then, the cells were grown at 28°C post-induction ON. Then, the cells were lysed and the proteins were purified by IMAC. Further, the different purification fractions were run on an SDS-gel to compare the amount of proteins eluted.
Further, a BCA assay was done on the purified proteins to quantify the yields.
Conclusion - It is very evident that switching the His-tag from the C-terminus to N-terminus increased the protein production of the Minispidroin protein substantially, by nearly 8 times.
Improvementes and Uses:
Improvements by Imperial-College 2024

Their designed basic part now consists of three major domains, the HRT2trunc; the NT domain which is this part; and an artifical hydrophobic surfactant protein (C33Leu) which mediates the formation of hydrophobic compartments.

Fig 1: the proposed micelle formation mechanism of this part. Created with Biorender.
Highlights of Results
We have investigated the formation of our artificial micelle using this part. This has been performed using a BODIPY staining technique, which the BODIPY molecule stains specifically with intracellular hydrophobic pockets via interactions with aliphatic polymers such as triglycerides and polyisoprene (rubber).

Fig.2: The fluorescent reading with excitation at 485nm and emission at 520nm for the cells stained with BODIPY. pET-28a(+) (pET) transformed BL21 induced with both 0mM and 1mM IPTG at OD600 = 0.5 serves as the global negative control (grey). The transformants of pET28a_HRT2trunc-NT-ASIP (ASIP) is induced with same IPTG conditions, where all cells are then cultured at 30oC for 16hr. The experiment is carried with 5 repeats of each sample, where the max and min from each group are represented by the error bars. The mean is shown with a cross, and the medium is identified as with the line inside the box. The box represents the interquartile range of the data distribution.
After rinsing the strained cells 3 times with PBSG (5% glycerol in PBS) to remove excess BODIPY molecule inside the cell membrane, the fluorescence of each group of cells were measured [7]. It was then observed that the HRT2trunc-NT-ASIP expressing cell (ASIP 1mM IPTG) produces significantly more fluorescence comparing to the non-induced transformant and negative controls. A one-tailed student t test was hence conducted to further compare the induced and non-induced groups, where solid statistical evidence was given with p<0.001 to further justify this observation. The average fluorescence of the induced cell is over 158% higher than the cells with no inducer added.
To further evident that our HRT2trunc-NT-ASIP is forming an artificial organelle that holds hydrophobic rubber molecule. We then imaged the BODIPY stained cells under a confocal microscope to identify the location of the stains inside the bacteria.

Fig.3 (Left) Positive group, HRT2trun-NT-ASIP with IPTG induced; (Right) Negative group, without being IPTG induced. Notice the yellow signal in the central of individual cells of IPTG induced sample. The samples were observed at 100x magnification.
The confocal microscopy images have revealed significantly higher and more nucleated fluorescence signal inside the HRT2trun-NT-ASIP expressing E. coli. Whereby the Z-stack images which scanned through the E. coli cell have evidenced that the signal cluster is present inside the cell rather attaching to the cell membrane. This visually evidenced that the ASIP is mediating the formation of artificial hydrophobic chamber inside the E. coli after translated. Since BODIPY selectively dyes hydrocarbons, it is then highly evidence that these organelles are very likely to carry natural rubber.
The part was also characterized for its ability to produce natural rubber. The E. coli expressing this part is cultured in large quantity (100ml) and induced overnight with 1mM IPTG at 30C with a pET negative control after reaching OD600 = 0.5. The cells were lysed through the chemical method (detailed at Imperial_college_2024 wiki, contribution page), where rubber is extracted using cyclohexane. The absorbance at the UV spectrum was then measured for the solutions.

Fig.4 (A): UV-vis absorbance curve of rubber extracted from the overnight cultures with cyclohexane. pET serves as a negative control. (B): comparing the absorbance value acquired at 210nm of different samples that contain natural rubber (cis-1,4-polyisoprene), ASIP is the HRT2trunc-NT-ASIP parts addressed in this registry.
The curve for ASIP is observed to be overall higher than the control group. Indicating a higher amount of polyisoprene being extracted with cyclohexane. The extract from cells expressing this part has also been compared with wild-type (pET28a) E. coli with known amounts of polyisoprene added. Through comparing the absorbance at 210nm a theoretical yield is thus determined to be around 0.1 to 0.5mg per 100ml of culture.