Difference between revisions of "Part:BBa K4632003"
(→Construction and Characterization) |
(→Construction and Characterization) |
||
| (22 intermediate revisions by the same user not shown) | |||
| Line 15: | Line 15: | ||
'''3. What we have done? (SCAU-China 2023)''' | '''3. What we have done? (SCAU-China 2023)''' | ||
| − | + | <p>The proteinase inhibitor CPTI was chosen, and OmpA was selected as the signal peptide. OmpA has been successfully employed for extracellular expression in prokaryotic systems during laboratory work (Pechsrichuang et al., 2016, Movva et al., 1980). We also obtained information from the Peking University iGEM team, indicating their successful experience using OmpA as a signal peptide (see HP Section). Therefore, using OmpA as a signal peptide for CPTI expression appeared to be a more promising approach (Figure 1)</p> | |
| − | + | ''https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/cpti-1.png'' | |
| + | <p><strong>Figure 1</strong> Diagram of CPTI circuit design</p> | ||
===Sequence and Features=== | ===Sequence and Features=== | ||
| Line 26: | Line 27: | ||
===Construction and Characterization=== | ===Construction and Characterization=== | ||
| + | |||
---- | ---- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | <p><strong>1. Verifying the Expression and excretion Proficiency of CPTI</strong></p> | ||
| + | <p>The OmpA -CPTI fragment (ordered from Guangzhou IGE Biotechnology Co.,Ltd.) was inserted into the pET-30a vector to generate the plasmid pET-30a-OmpA-CPTI. This plasmid was then transformed into ''E. coli'' BL21 and cultured overnight in LB medium. Overnight culture was dilute by 1/1000 to fresh LB medium, the IPTG was added when the OD600 reach 0.6. The medium was incubated another 3 hours after the ITPG addition, then centrifuged at 12,000 rpm 10 mins to obtain the supernatant and the precipitate. The supernatant was concentrated using ultrafiltration centrifuge tubes, while the precipitate was subjected to cell lysis with a lysis buffer. After lysis, the sample was centrifuged again to separate the supernatant from the post-lysis precipitate. The post-lysis precipitate was resuspended in lysis buffer. Tricine-PAGE (Figure 3) and Western Blot analyses (Figure 4) were performed on the above samples, and the results indicated that the CPTI protein was not expressed in ''E. coli''.</p> | ||
"https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/part-2-5.png" | "https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/part-2-5.png" | ||
| − | + | <p><strong>Figure 3</strong>. Tricine-PAGE analysis of CPTI expression. </p> | |
| − | <p><strong> Figure | + | <p>Lane 1: Concentrated supernatant of OmpA-CPTI (+IPTG); Lane 2: Concentrated supernatant of OmpA-CPTI (-IPTG); Lane 3: Concentrated supernatant of pET-30a(+IPTG); Lane 4: Whole cell lysate of OmpA-CPTI (+IPTG); Lane 5: Whole cell lysate of OmpA-CPTI (-IPTG); Lane 6: Whole cell lysate of CPTI (+IPTG); Lane 7: Whole cell lysateof of CPTI (-IPTG); Lane 8: Whole cell lysate of pET-30a (+IPTG); Lane 9: Whole cell lysate of pET-30a (-IPTG)</p> |
| − | Lane | + | |
| − | </p> | + | |
| − | + | ||
''https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/part-2-6.png'' | ''https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/part-2-6.png'' | ||
| − | <p><strong> Figure | + | <p><strong> Figure 4: </strong>Western blot analysis of CPTI expression</p> |
| − | + | <p>Lane 1: Concentrated supernatant of OmpA-CPTI (+IPTG); Lane 2: Concentrated supernatant of OmpA-CPTI (-IPTG); Lane 3: Concentrated supernatant of pET-30a(+IPTG); Lane 4: Whole cell lysate of OmpA-CPTI (+IPTG); Lane 5: Whole cell lysate of OmpA-CPTI (-IPTG); Lane 6: Whole cell lysate of CPTI (+IPTG); Lane 7: Whole cell lysate of of CPTI (-IPTG); Lane 8: Whole cell lysate of pET-30a (+IPTG); Lane 9: Whole cell lysate of pET-30a (-IPTG)</p> | |
| − | <p> | + | <p>With IPTG the cell could produce the detectable CPTI (Lane 4, Figure 4), however, the concentrated supernatant of CPTI could not been seen (Lane 1, Figure 4). This might because of the poor solution of CPTI with OmpA singlal peptide. To get a extracellular expressed CPTI, the GST tag, which could improve the solution of certain protein, was successfully added at the C-terminal of the CPTI gene. The protein induction expression experiment is currently underway.</p> |
| − | + | <p>This modification ensures that the protein can be expressed to a significant extent, as similar experiments have been reported (Yang et al., 2003). Our unique approach involves testing the activity of CPTI without removing the GST tag, which distinguishes our study from previous ones.</p> | |
| − | <p><strong> 2. Measurement of CPTI Activity</strong></p> | + | <p><strong>2. Measurement of CPTI Activity</strong></p> |
<p>The proteinase inhibitor failed to express successfully, as the intended proteinase inhibitor was a pancreatic trypsin inhibitor. The original plan was to determine the activity of the expressed proteinase inhibitor using the <strong>BAEE method</strong>, the principle of which is explained below: </p> | <p>The proteinase inhibitor failed to express successfully, as the intended proteinase inhibitor was a pancreatic trypsin inhibitor. The original plan was to determine the activity of the expressed proteinase inhibitor using the <strong>BAEE method</strong>, the principle of which is explained below: </p> | ||
Latest revision as of 14:23, 12 October 2023
Cowpea trypsin inhibitor(CPTI)
Description
1. How does it work? CPTI can inhibite the serine protease by interacting with the active site of serine protease. Simultaneously, CPTI inhibits insect feeding by stimulating feedback signals from insect (Xuhong-lin et al.,2008)
2. Eco-friendly and Safe
CPTI, a member of the Bowman-Birk protein family. Anti-insect spectrum tests show that CPTI inhibits almost all tested major agricultural pests. (Xuhong-lin et al.,2008) And, most importantly. it is Eco-friendly and Safe. (see more detail on[1])
3. What we have done? (SCAU-China 2023)
The proteinase inhibitor CPTI was chosen, and OmpA was selected as the signal peptide. OmpA has been successfully employed for extracellular expression in prokaryotic systems during laboratory work (Pechsrichuang et al., 2016, Movva et al., 1980). We also obtained information from the Peking University iGEM team, indicating their successful experience using OmpA as a signal peptide (see HP Section). Therefore, using OmpA as a signal peptide for CPTI expression appeared to be a more promising approach (Figure 1)
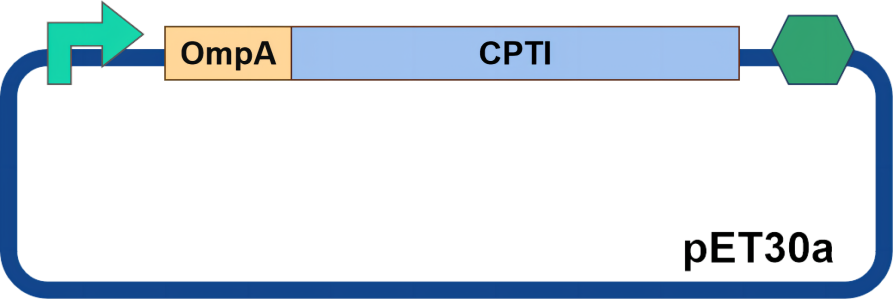
Figure 1 Diagram of CPTI circuit design
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Construction and Characterization
1. Verifying the Expression and excretion Proficiency of CPTI
The OmpA -CPTI fragment (ordered from Guangzhou IGE Biotechnology Co.,Ltd.) was inserted into the pET-30a vector to generate the plasmid pET-30a-OmpA-CPTI. This plasmid was then transformed into E. coli BL21 and cultured overnight in LB medium. Overnight culture was dilute by 1/1000 to fresh LB medium, the IPTG was added when the OD600 reach 0.6. The medium was incubated another 3 hours after the ITPG addition, then centrifuged at 12,000 rpm 10 mins to obtain the supernatant and the precipitate. The supernatant was concentrated using ultrafiltration centrifuge tubes, while the precipitate was subjected to cell lysis with a lysis buffer. After lysis, the sample was centrifuged again to separate the supernatant from the post-lysis precipitate. The post-lysis precipitate was resuspended in lysis buffer. Tricine-PAGE (Figure 3) and Western Blot analyses (Figure 4) were performed on the above samples, and the results indicated that the CPTI protein was not expressed in E. coli.
" "
"
Figure 3. Tricine-PAGE analysis of CPTI expression.
Lane 1: Concentrated supernatant of OmpA-CPTI (+IPTG); Lane 2: Concentrated supernatant of OmpA-CPTI (-IPTG); Lane 3: Concentrated supernatant of pET-30a(+IPTG); Lane 4: Whole cell lysate of OmpA-CPTI (+IPTG); Lane 5: Whole cell lysate of OmpA-CPTI (-IPTG); Lane 6: Whole cell lysate of CPTI (+IPTG); Lane 7: Whole cell lysateof of CPTI (-IPTG); Lane 8: Whole cell lysate of pET-30a (+IPTG); Lane 9: Whole cell lysate of pET-30a (-IPTG)

Figure 4: Western blot analysis of CPTI expression
Lane 1: Concentrated supernatant of OmpA-CPTI (+IPTG); Lane 2: Concentrated supernatant of OmpA-CPTI (-IPTG); Lane 3: Concentrated supernatant of pET-30a(+IPTG); Lane 4: Whole cell lysate of OmpA-CPTI (+IPTG); Lane 5: Whole cell lysate of OmpA-CPTI (-IPTG); Lane 6: Whole cell lysate of CPTI (+IPTG); Lane 7: Whole cell lysate of of CPTI (-IPTG); Lane 8: Whole cell lysate of pET-30a (+IPTG); Lane 9: Whole cell lysate of pET-30a (-IPTG)
With IPTG the cell could produce the detectable CPTI (Lane 4, Figure 4), however, the concentrated supernatant of CPTI could not been seen (Lane 1, Figure 4). This might because of the poor solution of CPTI with OmpA singlal peptide. To get a extracellular expressed CPTI, the GST tag, which could improve the solution of certain protein, was successfully added at the C-terminal of the CPTI gene. The protein induction expression experiment is currently underway.
This modification ensures that the protein can be expressed to a significant extent, as similar experiments have been reported (Yang et al., 2003). Our unique approach involves testing the activity of CPTI without removing the GST tag, which distinguishes our study from previous ones.
2. Measurement of CPTI Activity
The proteinase inhibitor failed to express successfully, as the intended proteinase inhibitor was a pancreatic trypsin inhibitor. The original plan was to determine the activity of the expressed proteinase inhibitor using the BAEE method, the principle of which is explained below:
The principle of the BAEE (Nα-Benzoyl-L-arginine ethyl ester hydrochloride) assay for measuring trypsin inhibitor (TI) activity is based on the ability of a pancreatic trypsin inhibitor to bind with pancreatic trypsin. BAEE is a substrate catalyzed by pancreatic trypsin, and the product of the catalytic reaction by pancreatic trypsin with BAEE absorbs light at a wavelength of 253 nm. The rate of increase in absorbance at A253 per unit time (min) represents the activity of pancreatic trypsin (U1). When pancreatic trypsin inhibitor (TI) is added to the reaction, TI inhibits the rate of production of the product in the BAEE reaction catalyzed by pancreatic trypsin, resulting in a slower rate of increase in absorbance at A253 per unit time (min). This reduced rate represents the residual activity of pancreatic trypsin after inhibition by TI (U2). Therefore, the activity of TI (TIA) can be calculated as follows: TIA = U1 - U2.
The activity of pancreatic trypsin (U1) is calculated as follows:
U1 = ΔA253/t / 0.001
The activity of pancreatic trypsin after inhibition by TI (U2) is calculated as follows:
U2 = ΔA253/t / 0.001
The activity of TI (TIA) can then be determined as:
TIA = U1 - U2
References
Xuhong-lin, Zhaihong-li, Wangfeng et al. Cowpea Trypsin Inhibitor Gene(cpti) and its Application in Insect Resistance Transgenic plants[J]. 2008.
Wei Gao, Feng Li, Qingyu Lu. CPTI (Cowpea Trypsin Inhibitor) gene, applications thereof and method for preparing great amount of CPTI: CN102127552B[P]. 2013-02-13.
