Difference between revisions of "Part:BBa I712019"
(→Characterization) |
(→Usage and Biology) |
||
| (28 intermediate revisions by 7 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_I712019 short</partinfo> | <partinfo>BBa_I712019 short</partinfo> | ||
| − | Firefly | + | Firefly Luciferase is luciferase from ''Photinus pyralis''. By oxidation of substrate luciferin light is produced. Luciferase thus acts as a reporter protein when connected to other proteins or promoters. |
| − | + | <h2>Characterization by Team Munich 2019</h2> | |
| − | In iGEM 2019, we the iGEM Munich team, characterised the | + | In iGEM 2019, we the iGEM Munich team, characterised the Firefly Luciferase. |
| − | ==Aim:== | + | ====Aim:==== |
| − | Determination of equilibrium parameters of the wild-type Firefly Luciferase (Part:BBa_I712019) in vivo in mammalian cells. | + | Determination of equilibrium parameters of the wild-type Firefly Luciferase (Part:BBa_I712019) ''in vivo'' in mammalian cells using the cell-permeable substrate, D-Luciferin. |
| − | Design: | + | ====Design:==== |
| − | + | As a first step, we ordered the sequence for the wild-type Firefly Luciferase (Part:BBa_I712019) and ligated it into a backbone under the control of a CAG promoter optimised for HEK293T cells (BBa_K3113200) using the Gibson Assembly technique. After sequencing confirmation, we transfected HEK293T cells with the plasmid containing the sequence for Firefly Luciferase (pCAG_FLuc). Finally, we performed a luciferase assay with different D-Luciferin concentrations and measured the luminescence with a plate reader. | |
| − | Materials and Measurement: | + | ====Materials and Measurement:==== |
- pCAG_FLuc | - pCAG_FLuc | ||
| Line 28: | Line 28: | ||
| − | HEK293T cells were transfected with pCAG_FLuc using our standardized protocol for transfection using Lipofectamine P3000. The cells were then grown for 48 hours at 37 °C and 5 % CO2 | + | HEK293T cells were transfected with pCAG_FLuc using our standardized protocol for transfection using Lipofectamine P3000. The cells were then grown for 48 hours at 37 °C and 5 % CO2. A dilution series of D-Luciferin was prepared ranging from 0 μM up to 8 mM, which was then added 1:1 to the transfected cells, reaching final concentrations from 0 μM up to 4 mM. As negative control we included samples where water instead of substrate was added. For the luciferase assay we used the GloMax® Discover Microplate Reader to measure the luminescence of our transfected cells. The assay was carried out at 37°C and no additional ATP or Mg2+ was added. For each substrate concentration we included four biological replicates, which were then averaged. D-Luciferin has a half-life of over 24 hours, allowing long-term measurements. Therefore, we measured our samples every five minutes over a period of approximately 8 hours, gaining more data for technical replicates. |
| + | ====Analysis:==== | ||
| + | All the analyses were performed using Graphpad Prism (San Diego, CA). | ||
| − | Results: | + | ====Results:==== |
| − | Time-resolved data of biological replicates for the | + | Time-resolved data of biological replicates for the luciferase assay are summarized in Figure 1. We observed a concentration dependency of Firefly Luciferase in regard to D-Luciferin. |
| − | + | ||
| − | + | ||
<html> | <html> | ||
<figure class="figure"> | <figure class="figure"> | ||
| − | <img src="https://2019.igem.org/wiki/images/0/01/T--Munich--Charac_diff_conc_V2.png" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure." height=" | + | <img src="https://2019.igem.org/wiki/images/0/01/T--Munich--Charac_diff_conc_V2.png" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure." height="200px"> |
| − | <figcaption class="figure-caption"> Figure 1 | + | <figcaption class="figure-caption"> Figure 1: Luciferase Assay to determine equilibrium binding constant of the Firefly Luciferase and Luciferin. A dilution series of D-Luciferin was prepared reaching final concentrations from 0 μM up to 4 mM of D-Luciferin and luminescence was measured for ~8h. |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| + | </html> | ||
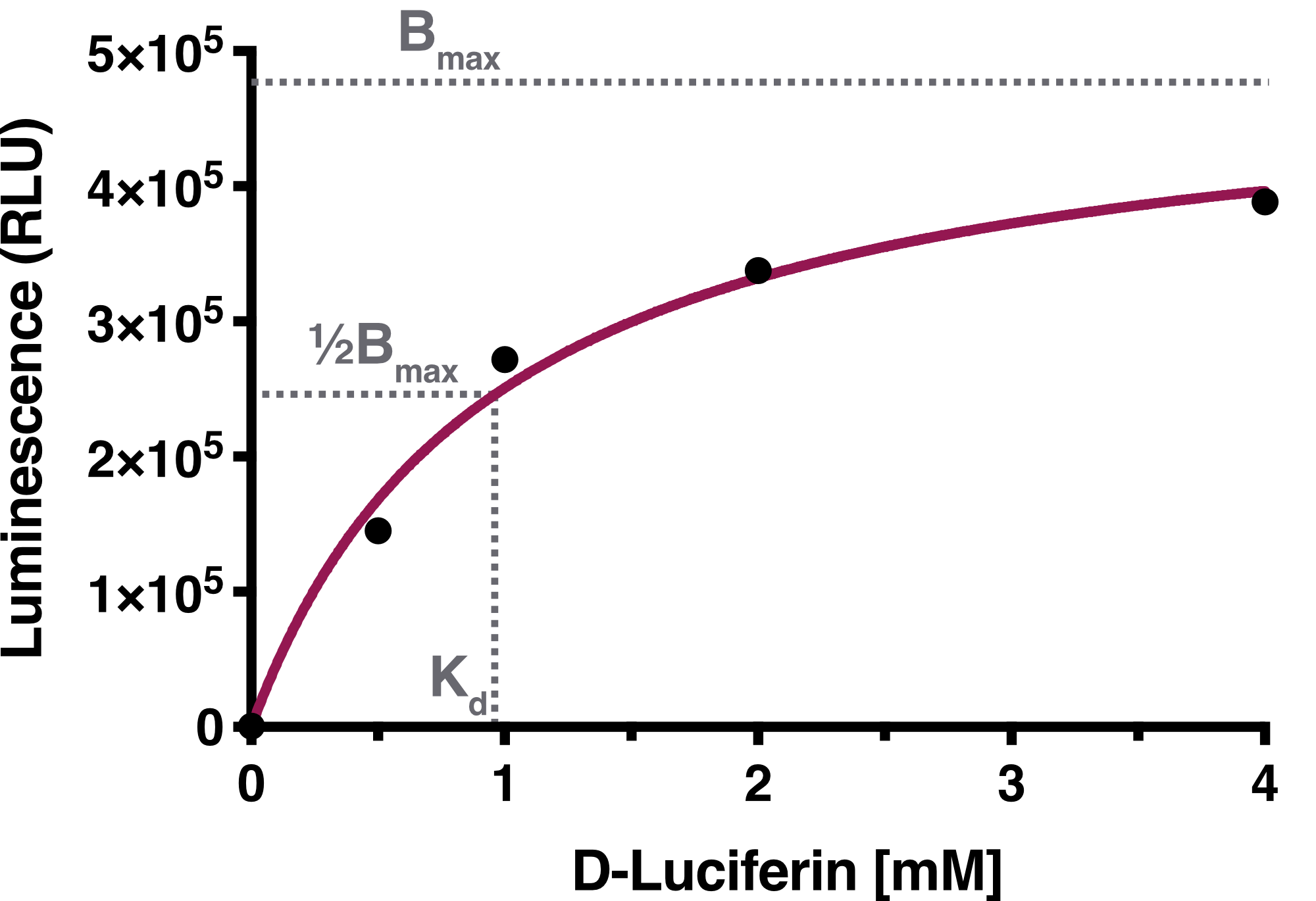
| + | We determined the equilibrium binding constant, Kd (D-Luciferin), by plotting the averaged equilibrium values against the used D-Luciferin concentrations. The calculated equilibrium binding constant Kd (D-Luciferin) equals 0.9619 mM, representing the D-Luciferin concentration needed to achieve half-maximum binding at equilibrium. The solid line represents the fit used for the determination of the equilibrium binding constant. | ||
| + | <html> | ||
<figure class="figure"> | <figure class="figure"> | ||
| − | <img src="https://2019.igem.org/wiki/images/8/8d/T--Munich--Charac_binding_curve.png" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure." height=" | + | <img src="https://2019.igem.org/wiki/images/8/8d/T--Munich--Charac_binding_curve.png" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure." height="200px"> |
| − | <figcaption class="figure-caption"> Figure 2 | + | <figcaption class="figure-caption"> Figure 2: The various D-Luciferin concentrations used throughout the luciferase assay were plotted against the averaged equilibrium values. |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 57: | Line 60: | ||
</html> | </html> | ||
| + | ====Discussion:==== | ||
| − | + | By following the luminescence signal over a longer time period and including four biological replicates, we obtained consistent data. We aimed to standardize this assay by establishig a standart curve with purified Firefly Luciferase from ''E.coli'', but unfortunately we weren't able to successfully purify FLuc overexpressed from ''E.coli'' cells. However, we standardized our assay by seeding the same amount of cells into each well and transfecting them with the same concentration of DNA and included four biological replicates for each condition tested. | |
| − | + | <h2>Modification</h2> | |
| − | + | ||
| − | + | ||
In iGEM 2016, our team, SYSU-MEDICINE, had constructed a new composite part [https://parts.igem.org/Part:BBa_K1993006 BBa_K1993006] in order to improve the positioning function of this previous part. Our new composite part contains gene Firefly Luciferase, dTomato and hFTH, which help users observe biological processes in vivo and in vitro. | In iGEM 2016, our team, SYSU-MEDICINE, had constructed a new composite part [https://parts.igem.org/Part:BBa_K1993006 BBa_K1993006] in order to improve the positioning function of this previous part. Our new composite part contains gene Firefly Luciferase, dTomato and hFTH, which help users observe biological processes in vivo and in vitro. | ||
| Line 85: | Line 87: | ||
For more information, please see our Part page: [https://parts.igem.org/Part:BBa_K1993006 https://parts.igem.org/Part:BBa_K1993006 ] | For more information, please see our Part page: [https://parts.igem.org/Part:BBa_K1993006 https://parts.igem.org/Part:BBa_K1993006 ] | ||
| + | In our project (iGEM 2023, Nanjing-NFLS), we spliced the firefly luciferase gene behind our target gene segment. By co-transfecting them into recipient cells and detecting the luciferase activity, we can speculate whether the genes under test are successfully expressed. | ||
| + | For more information, please see our Part page: https://parts.igem.org/Part:BBa_K4606011 | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 95: | Line 99: | ||
| − | < | + | {{SDSZ_China}} |
| − | === | + | <html> |
| − | < | + | |
| − | < | + | |
| + | |||
| + | |||
| + | <div class="clear"></div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <div class="column full_size"> | ||
| + | |||
| + | <h2>Characterization by SDSZ_China 2019</h2> | ||
| + | |||
| + | |||
| + | <p style="color:black;font-size:15px;"> | ||
| + | We are Team SDSZ_China in iGEM2019, and we used this part to detect bacteriophage density in fecal polluted water. When different phage densities (1x, 2x, 5x, 10x ideally) were prepared and substrate solution (D-luciferin, MgSO4, ATP) was added to the liquid LB culture of E.coli. solution, cell lysis occur and the expressed enzymes would release to react with their substrate to emit bioluminescence. | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | 1. Model | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | We have documented the OD values when conducting IPTG induction to get the best level of protein expression. As induction proceeds and OD600 value reaches somewhat near 0.3, protein expression is tested to be at high level by mathematical model. | ||
| + | </p> | ||
| + | <img src="https://2019.igem.org/wiki/images/3/39/T--SDSZ_China--a.jpeg" alt=" width="500" height="500"" > | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | Note:the OD values to get the best level of protein expression are determined by analyzing the data and standard transformations. | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | 2. Solution and reaction | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | Through various tests and professional journals, we have prepared effective substrate solution for our detection system listed as below: | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | Mg2+ 0.001mol/L | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | ATP 0.001mol/L | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | D-luciferin 5mg | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | E.coli. solution with OD value around 0.3 500ul | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | Add liquid LB medium to the whole reaction system until volume reaches 3000ul. | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | Under this substrate system, the reaction went on smoothly when bacteriophages were introduced to the solution. | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | 3. Results | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | As we added phage solutions to the culture medium, bioluminescence was observed and recorded as below. | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | figure under natural light | figure under ultraviolet light | ||
| + | </p> | ||
| + | <img src="https://2019.igem.org/wiki/images/6/6b/T--SDSZ_China--dd.jpeg" class="rounded mx-auto d-block" alt=" width="500" height="500""> | ||
| + | </br> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | -From left to right: | ||
| + | </p> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | negative control | 5x coliphage solution | 2x coliphage solution | ||
| + | </p> | ||
| + | <img src="https://2019.igem.org/wiki/images/d/d8/T--SDSZ_China--cc.png" class="rounded mx-auto d-block" alt="..." width="50%" height="50%" style="Padding:0px"> | ||
| + | <p style="color:black;font-size:15px;"> | ||
| + | Note: Since there is no proper device in our lab for observing bioluminescence, the observation and documentation of our detection result went on rather difficultly. Although D-luciferin may seem to be faintly luminescence under ultraviolet light, we confirmed that it does not emit any observable luminescence in complete darkness. | ||
| + | </p> | ||
| + | </div> | ||
| + | </html> | ||
Latest revision as of 12:33, 11 October 2023
Firefly luciferase - luciferase from Photinus pyralis
Firefly Luciferase is luciferase from Photinus pyralis. By oxidation of substrate luciferin light is produced. Luciferase thus acts as a reporter protein when connected to other proteins or promoters.
Characterization by Team Munich 2019
In iGEM 2019, we the iGEM Munich team, characterised the Firefly Luciferase.
Aim:
Determination of equilibrium parameters of the wild-type Firefly Luciferase (Part:BBa_I712019) in vivo in mammalian cells using the cell-permeable substrate, D-Luciferin.
Design:
As a first step, we ordered the sequence for the wild-type Firefly Luciferase (Part:BBa_I712019) and ligated it into a backbone under the control of a CAG promoter optimised for HEK293T cells (BBa_K3113200) using the Gibson Assembly technique. After sequencing confirmation, we transfected HEK293T cells with the plasmid containing the sequence for Firefly Luciferase (pCAG_FLuc). Finally, we performed a luciferase assay with different D-Luciferin concentrations and measured the luminescence with a plate reader.
Materials and Measurement:
- pCAG_FLuc
- HEK293T cells
- D-Luciferin (100 mM stock solution)
- GloMax® Discover Microplate Reader (Promega)
HEK293T cells were transfected with pCAG_FLuc using our standardized protocol for transfection using Lipofectamine P3000. The cells were then grown for 48 hours at 37 °C and 5 % CO2. A dilution series of D-Luciferin was prepared ranging from 0 μM up to 8 mM, which was then added 1:1 to the transfected cells, reaching final concentrations from 0 μM up to 4 mM. As negative control we included samples where water instead of substrate was added. For the luciferase assay we used the GloMax® Discover Microplate Reader to measure the luminescence of our transfected cells. The assay was carried out at 37°C and no additional ATP or Mg2+ was added. For each substrate concentration we included four biological replicates, which were then averaged. D-Luciferin has a half-life of over 24 hours, allowing long-term measurements. Therefore, we measured our samples every five minutes over a period of approximately 8 hours, gaining more data for technical replicates.
Analysis:
All the analyses were performed using Graphpad Prism (San Diego, CA).
Results:
Time-resolved data of biological replicates for the luciferase assay are summarized in Figure 1. We observed a concentration dependency of Firefly Luciferase in regard to D-Luciferin.

We determined the equilibrium binding constant, Kd (D-Luciferin), by plotting the averaged equilibrium values against the used D-Luciferin concentrations. The calculated equilibrium binding constant Kd (D-Luciferin) equals 0.9619 mM, representing the D-Luciferin concentration needed to achieve half-maximum binding at equilibrium. The solid line represents the fit used for the determination of the equilibrium binding constant.
Discussion:
By following the luminescence signal over a longer time period and including four biological replicates, we obtained consistent data. We aimed to standardize this assay by establishig a standart curve with purified Firefly Luciferase from E.coli, but unfortunately we weren't able to successfully purify FLuc overexpressed from E.coli cells. However, we standardized our assay by seeding the same amount of cells into each well and transfecting them with the same concentration of DNA and included four biological replicates for each condition tested.
Modification
In iGEM 2016, our team, SYSU-MEDICINE, had constructed a new composite part BBa_K1993006 in order to improve the positioning function of this previous part. Our new composite part contains gene Firefly Luciferase, dTomato and hFTH, which help users observe biological processes in vivo and in vitro.
For more information, please see our Part page: https://parts.igem.org/Part:BBa_K1993006
Improvement of the function
This part was improved by NUDT_CHINA in iGEM 2017 to optimize its function in a dual-luciferase miRNA target expression system. Two restriction sites of BsmBI were added to the 3’ end of firefly luciferase, through which, the 3’ untranslated region of any gene can be inserted using Golden-Gate Assembly, with no worry about the multiple illegal sites contained in it. For more information, please visit our part page: https://parts.igem.org/Part:BBa_K2440008.
Usage and Biology
This part contains a Eukaryotic ribosome binding site and non-standard prefix and suffix. See the sequence first.
We used the part BBa_I712019 to construct the part with split-luciferase. Our sequencing result suggested that this part is completely working. --------Tianjin IGEM TEAM
In iGEM 2016, our team, SYSU-MEDICINE, had constructed a new composite part BBa_K1993006 in order to improve the positioning function of this previous part. Our new composite part contains gene Firefly Luciferase, dTomato and hFTH, which help users observe biological processes in vivo and in vitro.
For more information, please see our Part page: https://parts.igem.org/Part:BBa_K1993006
In our project (iGEM 2023, Nanjing-NFLS), we spliced the firefly luciferase gene behind our target gene segment. By co-transfecting them into recipient cells and detecting the luciferase activity, we can speculate whether the genes under test are successfully expressed. For more information, please see our Part page: https://parts.igem.org/Part:BBa_K4606011
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 808
Characterization by SDSZ_China 2019
We are Team SDSZ_China in iGEM2019, and we used this part to detect bacteriophage density in fecal polluted water. When different phage densities (1x, 2x, 5x, 10x ideally) were prepared and substrate solution (D-luciferin, MgSO4, ATP) was added to the liquid LB culture of E.coli. solution, cell lysis occur and the expressed enzymes would release to react with their substrate to emit bioluminescence.
1. Model
We have documented the OD values when conducting IPTG induction to get the best level of protein expression. As induction proceeds and OD600 value reaches somewhat near 0.3, protein expression is tested to be at high level by mathematical model.
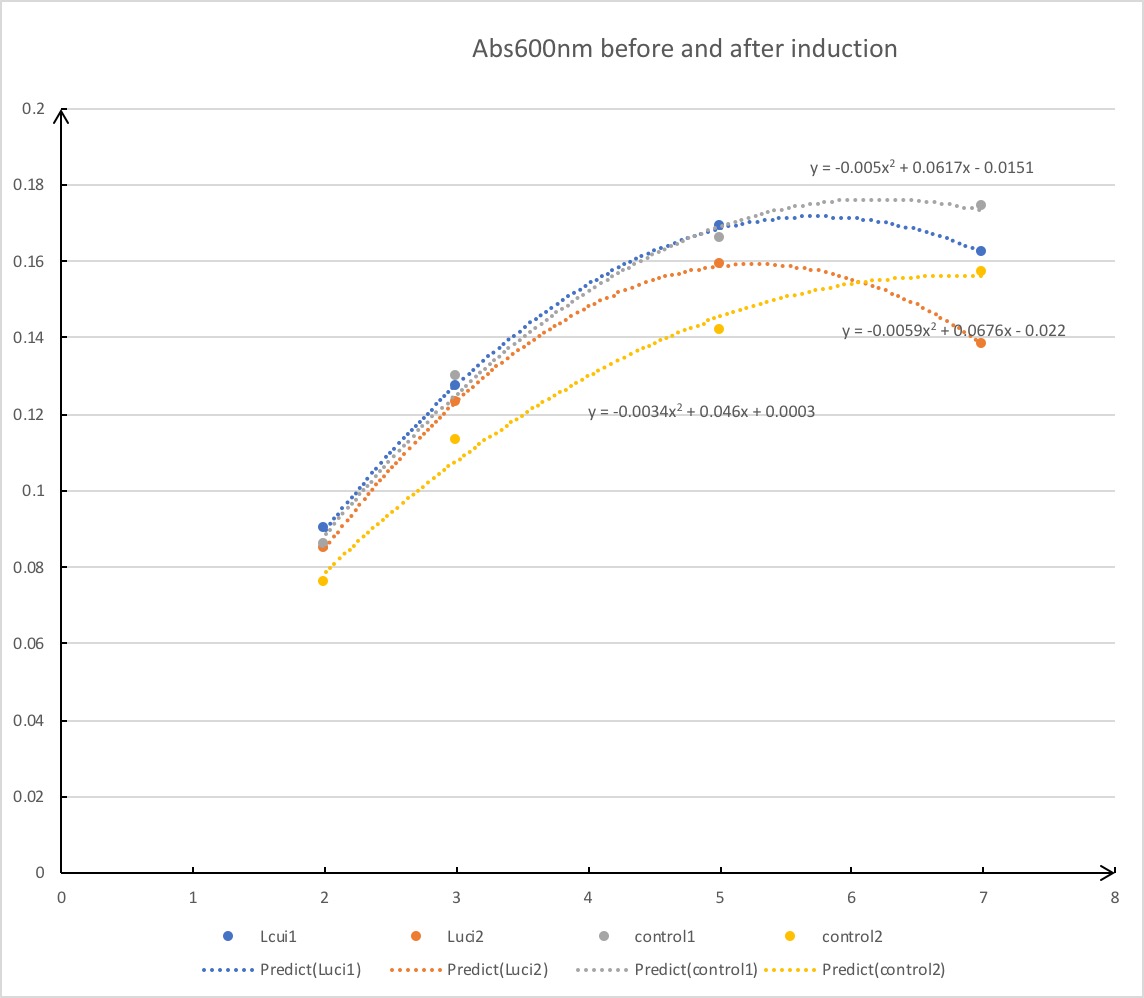
Note:the OD values to get the best level of protein expression are determined by analyzing the data and standard transformations.
2. Solution and reaction
Through various tests and professional journals, we have prepared effective substrate solution for our detection system listed as below:
Mg2+ 0.001mol/L
ATP 0.001mol/L
D-luciferin 5mg
E.coli. solution with OD value around 0.3 500ul
Add liquid LB medium to the whole reaction system until volume reaches 3000ul.
Under this substrate system, the reaction went on smoothly when bacteriophages were introduced to the solution.
3. Results
As we added phage solutions to the culture medium, bioluminescence was observed and recorded as below.
figure under natural light | figure under ultraviolet light
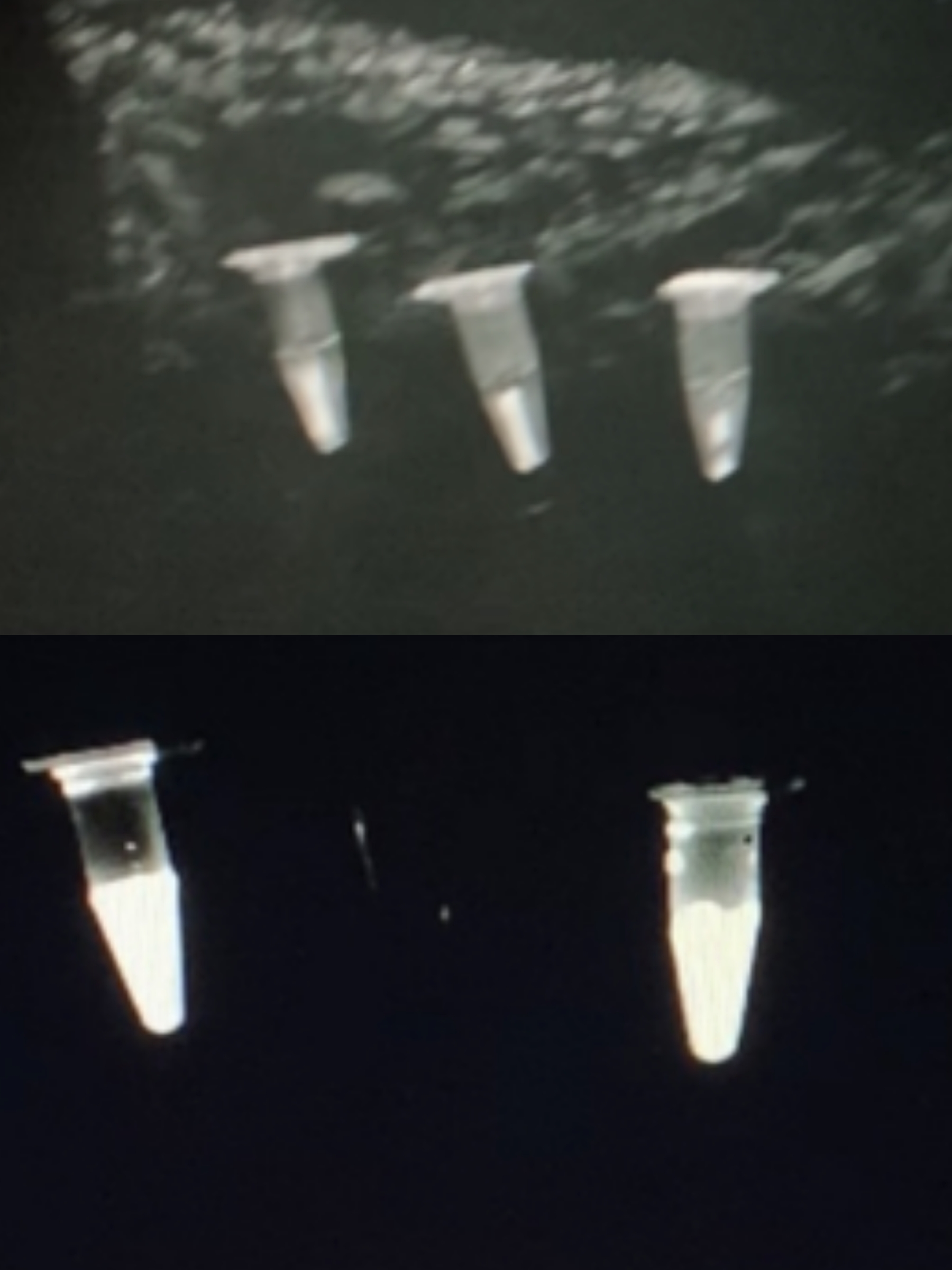
-From left to right:
negative control | 5x coliphage solution | 2x coliphage solution

Note: Since there is no proper device in our lab for observing bioluminescence, the observation and documentation of our detection result went on rather difficultly. Although D-luciferin may seem to be faintly luminescence under ultraviolet light, we confirmed that it does not emit any observable luminescence in complete darkness.
