Difference between revisions of "Part:BBa K112806"
(→Team NYMU-Taipei 2017: Successfully construct T4 endolysin into suicide mechanism) |
(→Contribution of SCAU-China 2023) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
The lysozyme from enterobacteria phage T4 degrades peptidoglycan layer. | The lysozyme from enterobacteria phage T4 degrades peptidoglycan layer. | ||
| − | Group: (Michigan 2017) Author: (Aaron Renberg) Summary: We improved part BBa_K112806 from UC Berkeley’s 2008 project by optimizing the codons for translation in E. coli using IDT’s codon optimization tool making it much easier for future iGEM teams to use. The changes we made were T508C and A511G. Link: https://parts.igem.org/Part:BBa_K2301001 | + | Group: (Michigan 2017) Author: (Aaron Renberg) Summary: We improved part BBa_K112806 from UC Berkeley’s 2008 project by optimizing the codons for translation in E. coli using IDT’s codon optimization tool making it much easier for future iGEM teams to use. The changes we made were T508C and A511G. Additionally, we constructed three different versions (of varying promoter strength) of a temperature controlled kill switch using holin, endolysin and antiholin. Link: https://parts.igem.org/Part:BBa_K2301001 |
| Line 69: | Line 69: | ||
[[File:T--NYMU-Taipei--TAS-FunctionalTest.png|600px]] | [[File:T--NYMU-Taipei--TAS-FunctionalTest.png|600px]] | ||
| + | |||
| + | |||
| + | ==Contribution of SCAU-China 2023== | ||
| + | '''What have we done? ''' | ||
| + | |||
| + | <p>To achieve control over toxicant concentration, we introduced components labeled with '''BBa_K112806'''[https://parts.igem.org/Part:BBa_K112806] to create the T4-T4 lysis device. </p> | ||
| + | |||
| + | <p>We characterized the component to demonstrate its effectiveness, as detailed in the construction and characterization section.</p> | ||
| + | |||
| + | |||
| + | '''1. Construction''' | ||
| + | |||
| + | In our initial validation experiments, we utilized a dual-plasmid system consisting of pBAD24M and pBAD33 to test our device. (Plasmid maps can be found in Figures 1) | ||
| + | <p>First, both sets of plasmids were co-transformed into ''E. coli'' TOP10 using the KCM ice-cold method, and the success of transformation was confirmed using PCR.</p> | ||
| + | |||
| + | <p>Next, the successfully transformed engineered bacteria were streaked onto agar plates and incubated at 37 degrees Celsius. Single clones were selected and inoculated into liquid LB medium. Inducer was added, and the cultures were grown for 6 hours.</p> | ||
| + | |||
| + | |||
| + | ''https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/cp-1-1-2.png'' | ||
| + | <p><strong>Fig.1</strong> Diagram of the quorum sensing-based T4 lysis device circuit design</p> | ||
| + | |||
| + | |||
| + | '''2. Validation of Product Expression Level Control''' | ||
| + | |||
| + | |||
| + | '''(1)Pre-experiment for Induced Expression of Lysis Effect''' | ||
| + | The successfully transformed engineered bacteria were streaked on plates and grown at 37°C. Single colonies were picked and inoculated into liquid LB medium, followed by the addition of inducers. The cultures were incubated for 6 hours. | ||
| + | |||
| + | ''https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/cp-5.png'' | ||
| + | <p><strong>Fig.2</strong> Verification of lysis effect</p> | ||
| + | <p>The blank control was LB with 20% Ara. The control group consisted of the engineered bacteria without inducer, while the experimental group consisted of the engineered bacteria with a final concentration of 0.02% Ara. Each group had 3 replicates.</p> | ||
| + | |||
| + | '''Result''' | ||
| + | <p>After zeroing with the blank control, it was observed that the bacterial density in the experimental group decreased to 34.3% of that in the control group, indicating a significant lysis effect. This confirms the successful expression of quorum sensing and initiation of downstream lysis gene expression. Lysis gene expression was successful without leakage.</p> | ||
| + | |||
| + | |||
| + | '''(2)Growth Curve Testing of the Second Verification System''' | ||
| + | |||
| + | <p>Single clones were selected and inoculated into LB medium. After overnight incubation, the OD600 was adjusted to 0.6, and arabinose was added to a final concentration of 0.02%. The cultures were shaken for 21 hours in a sterile 96-well plate, and a growth curve was plotted. | ||
| + | The blank control group was LB broth, the control group was wild Top10, and the experimental group was the engineering bacteria consisting of pBAD24M and pBAD33. There were 6 replicates per group.</p> | ||
| + | |||
| + | ''https://static.igem.wiki/teams/4632/wiki/wiki/registry-part/cp-1-3-1.png'' | ||
| + | <p><strong>Fig.3</strong> Growth curve testing of the quorum sensing-based T4 lysis device</p> | ||
| + | |||
| + | |||
| + | |||
| + | '''Result'''<p> The growth curve revealed that the bacterial density continued to rise in the first 4 hours and began to decrease after 4 hours, stabilizing around the 9th hour. In contrast, the control group's bacterial density continued to rise. </p> | ||
| + | |||
| + | <p>At the 4th hour, the quorum sensing signal reached the threshold, initiating lysis gene expression. The engineered bacteria lysed, resulting in a significant decrease in bacterial density, which stabilized around the 9th hour.</p> More detail link: https://parts.igem.org/Part:BBa_K4632024 | ||
Latest revision as of 14:03, 10 October 2023
[T4 endolysin]
The lysozyme from enterobacteria phage T4 degrades peptidoglycan layer.
Group: (Michigan 2017) Author: (Aaron Renberg) Summary: We improved part BBa_K112806 from UC Berkeley’s 2008 project by optimizing the codons for translation in E. coli using IDT’s codon optimization tool making it much easier for future iGEM teams to use. The changes we made were T508C and A511G. Additionally, we constructed three different versions (of varying promoter strength) of a temperature controlled kill switch using holin, endolysin and antiholin. Link: https://parts.igem.org/Part:BBa_K2301001
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 271
Illegal AgeI site found at 341 - 1000COMPATIBLE WITH RFC[1000]
Team NYMU-Taipei 2017: Successfully construct T4 endolysin into suicide mechanism
Improvement
We successfully combine T4 endolysin with a constitutive promoter (BBa_J23106), a ribosome binding site (BBa_B0034) and a double terminator (BBa_B0010 and BBa_B0012). Furthermore, we construct T4 holin and T4 endolysin together as a functional suicide mechanism. Besides, in NYMU-Taipei 2017 team’s project, we also put the suicide mechanism into practice by constructing them with NrtA.
Result
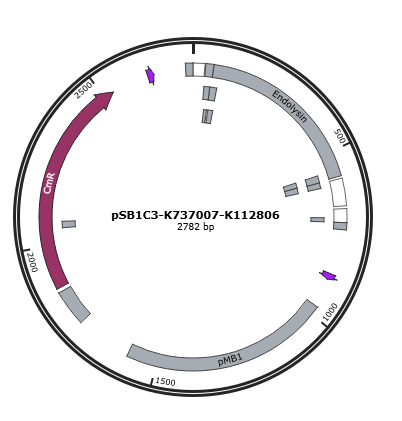
Figure 1 is the gene map of T4 endolysin construct, pSB1C3-K737007-K112806.
Figure 1
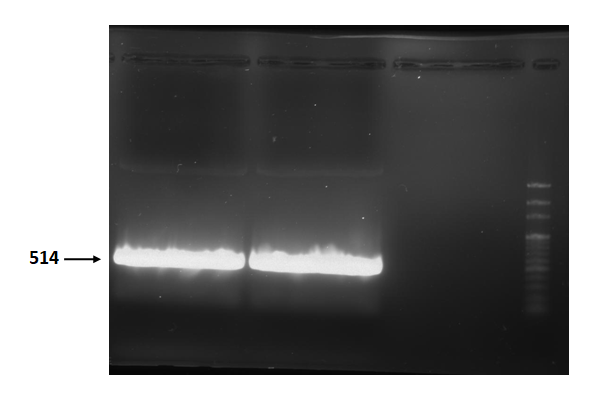
Figure 2 is electrophoresis result of endolysin (from BBa_K112806) PCR product.
The marker is 100bp. The length is 514bp as expected.
Figure 2
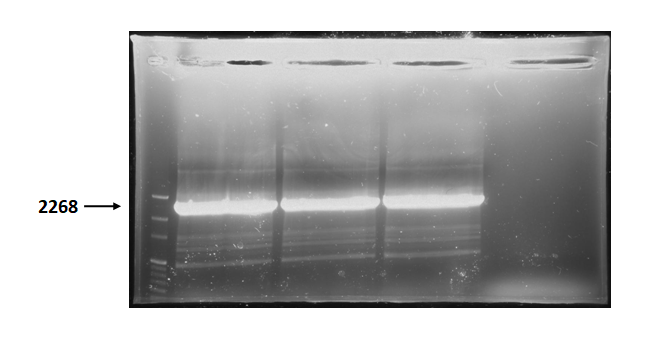
Figure 3 is electrophoresis result of endolysin backbone (from BBa_K737007) PCR product.
The marker is 100bp. The length is 2268bp as expected.
Figure 3
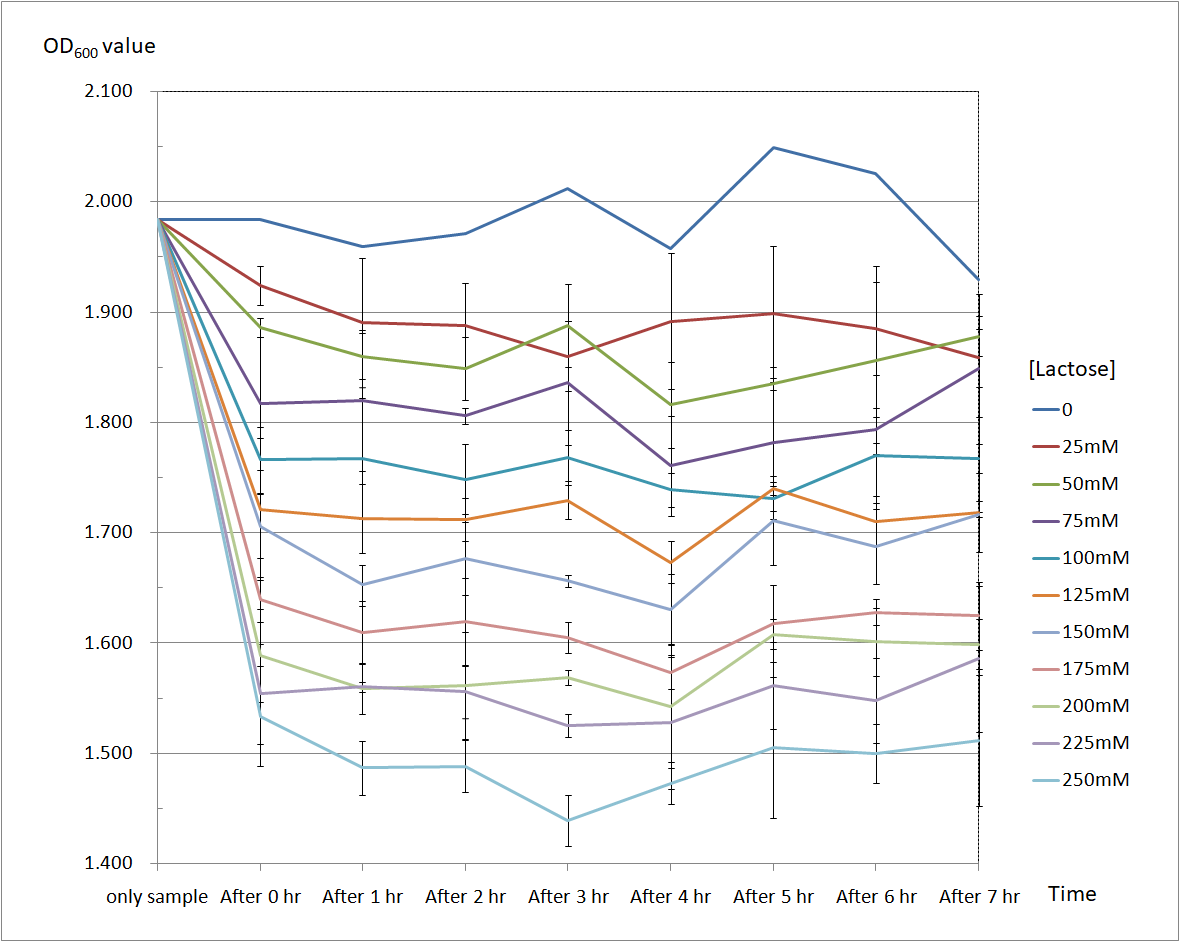
Figure 4 and Figure 5 are the results of suicide mechanism functional test.
Both figure show that our suicide mechanism can be induced by adding lactose, and the effectiveness of suicide mechanism goes better as the concentration of lactose goes higher.
Figure 4 is the bacterium with holin-endolysin construct.
As figure 3 shows, the suicide mechanism is induced immediately when lactose is added into the samples. Besides, we can see that the lactose concentration and the OD value of the bacterium with holin-endolysin construct are positively correlated, which means our suicide mechanism does work.
Figure 4
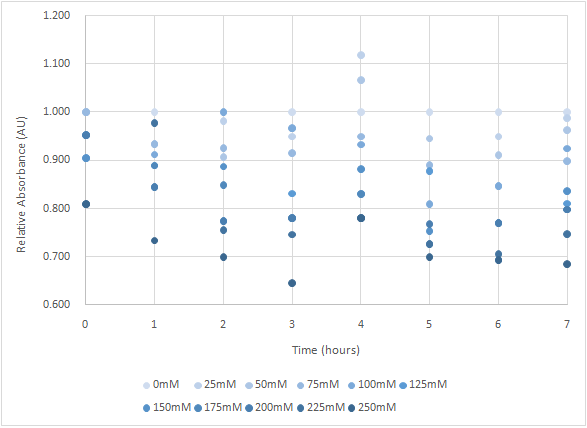
Figure 5 is the bacterium with holin-endolysin-NrtA construct.
As figure 4 shows, the trend of the relative absorbance is downward as the lactose is added to induce the suicide mechanism. The concentration of lactose is also positively correlated with the declining degree of relative absorbance.
Figure 5
Contribution of SCAU-China 2023
What have we done?
To achieve control over toxicant concentration, we introduced components labeled with BBa_K112806[1] to create the T4-T4 lysis device.
We characterized the component to demonstrate its effectiveness, as detailed in the construction and characterization section.
1. Construction
In our initial validation experiments, we utilized a dual-plasmid system consisting of pBAD24M and pBAD33 to test our device. (Plasmid maps can be found in Figures 1)
First, both sets of plasmids were co-transformed into E. coli TOP10 using the KCM ice-cold method, and the success of transformation was confirmed using PCR.
Next, the successfully transformed engineered bacteria were streaked onto agar plates and incubated at 37 degrees Celsius. Single clones were selected and inoculated into liquid LB medium. Inducer was added, and the cultures were grown for 6 hours.

Fig.1 Diagram of the quorum sensing-based T4 lysis device circuit design
2. Validation of Product Expression Level Control
(1)Pre-experiment for Induced Expression of Lysis Effect
The successfully transformed engineered bacteria were streaked on plates and grown at 37°C. Single colonies were picked and inoculated into liquid LB medium, followed by the addition of inducers. The cultures were incubated for 6 hours.

Fig.2 Verification of lysis effect
The blank control was LB with 20% Ara. The control group consisted of the engineered bacteria without inducer, while the experimental group consisted of the engineered bacteria with a final concentration of 0.02% Ara. Each group had 3 replicates.
Result
After zeroing with the blank control, it was observed that the bacterial density in the experimental group decreased to 34.3% of that in the control group, indicating a significant lysis effect. This confirms the successful expression of quorum sensing and initiation of downstream lysis gene expression. Lysis gene expression was successful without leakage.
(2)Growth Curve Testing of the Second Verification System
Single clones were selected and inoculated into LB medium. After overnight incubation, the OD600 was adjusted to 0.6, and arabinose was added to a final concentration of 0.02%. The cultures were shaken for 21 hours in a sterile 96-well plate, and a growth curve was plotted. The blank control group was LB broth, the control group was wild Top10, and the experimental group was the engineering bacteria consisting of pBAD24M and pBAD33. There were 6 replicates per group.

Fig.3 Growth curve testing of the quorum sensing-based T4 lysis device
The growth curve revealed that the bacterial density continued to rise in the first 4 hours and began to decrease after 4 hours, stabilizing around the 9th hour. In contrast, the control group's bacterial density continued to rise.
At the 4th hour, the quorum sensing signal reached the threshold, initiating lysis gene expression. The engineered bacteria lysed, resulting in a significant decrease in bacterial density, which stabilized around the 9th hour.
More detail link: https://parts.igem.org/Part:BBa_K4632024