Difference between revisions of "Part:BBa K4380014"
(→Experimental characterisation) |
(→Directed evolution) |
||
| (30 intermediate revisions by the same user not shown) | |||
| Line 16: | Line 16: | ||
==Usage== | ==Usage== | ||
| − | Bacterial display systems are routinely used for the improvement of antibodies, peptides, or enzymes. One frequently used bacterial cell surface display system is based on the EstA protein. Although there are different approaches | + | Bacterial display systems are routinely used for the improvement of antibodies, peptides, or enzymes. One frequently used bacterial cell surface display system is based on the EstA protein. Although there are different approaches to using this protein for cell surface display exposure, one of the most frequent uses involves a mutation at active residue <b>S38A</b>. This mutation eliminates enzymes esterase activity but the enzyme is still able to be expressed on bacterial surfaces, therefore, can be used as an anchoring motif. The catalytically inactivated extracellular domain and the passenger domain are subsequently translocated to the cell surface [1][3] and ''E. coli'' cells can heterologously present approximately 36,000 copies of EstA on the cell surface [1][6]. |
===Directed evolution=== | ===Directed evolution=== | ||
| − | ''E. coli'' cells present approximately 36,000 copies of EstA on the cell surface [1]. Therefore, high presence on bacterial surfaces (especially, when recombinant proteins can be expressed in high levels), is suitable for <b> | + | ''E. coli'' cells present approximately 36,000 copies of EstA on the cell surface [1]. Therefore, high presence on bacterial surfaces (especially, when recombinant proteins can be expressed in high levels), is suitable for <b>direct evolution approaches</b>. Direct Evolution (DE) is a method used in protein engineering that mimics the process of natural selection to steer proteins or nucleic acids toward a user-defined goal [4]. This method has successfully transformed the way we view protein engineering. During ''in vivo'' evolution, each cell is transformed with a plasmid containing a different member of the variant library. Protein EstA allows the expression of library variants on its surface, where its function can be tested. Therefore, enzymes that are presented on the cell surface or are secreted to the periplasm can successfully be engineered by directed evolution [2]. With this in mind, these systems are routinely used for the improvement of antibodies, peptides, enzymes, or nanobodies [3][4][5]. |
==Experimental characterisation== | ==Experimental characterisation== | ||
===Cell surface display prooval=== | ===Cell surface display prooval=== | ||
====Proteinase K assay==== | ====Proteinase K assay==== | ||
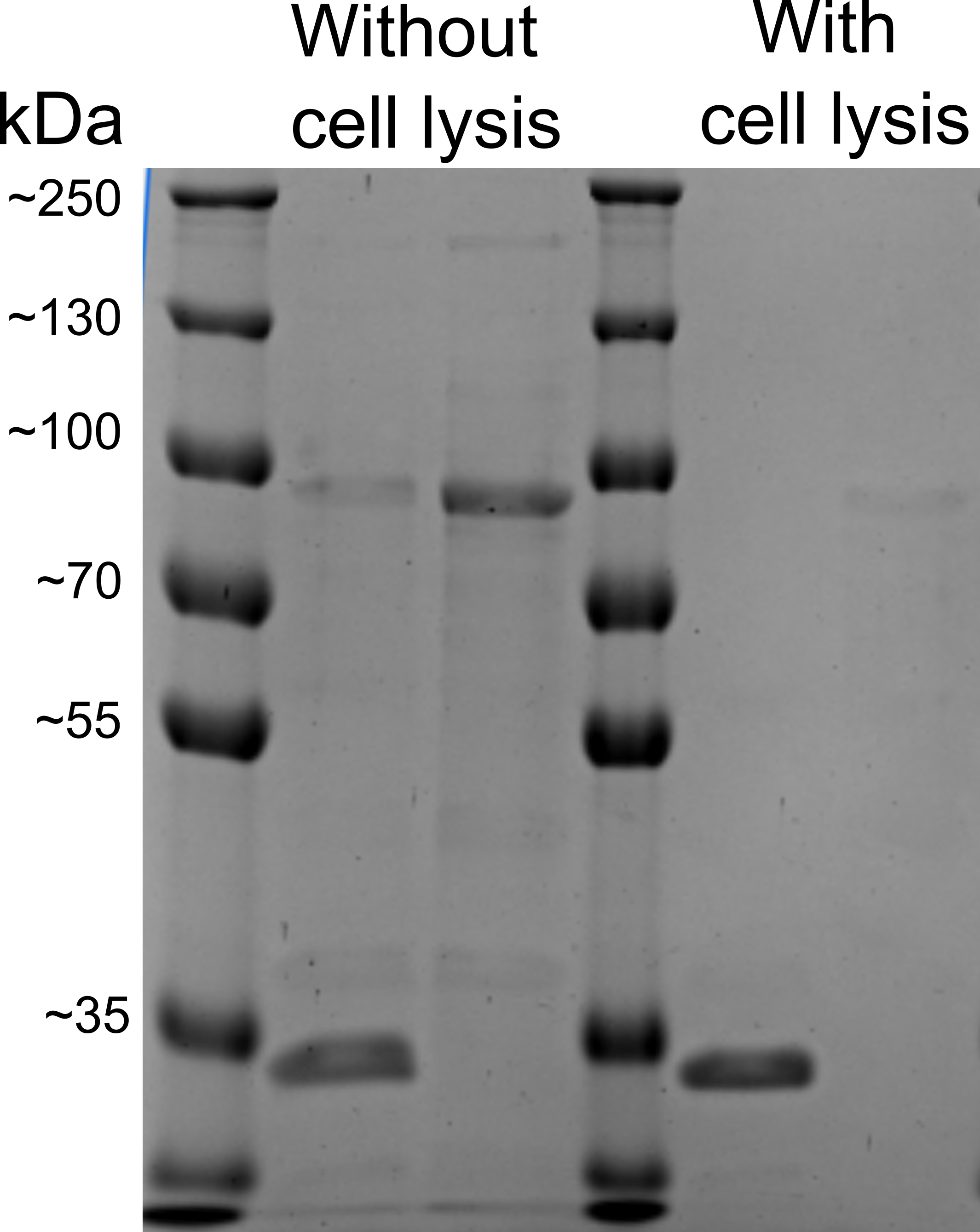
| − | To first characterize the protein, used for this assay (EstA), we decided to test, whether our protein is expressed onto bacterial membrane. | + | [[File:pK.gif|200px|thumb|right|Figure 1: Proteinase K assay performed on cell surface display protein EstA with and without bacterial lysis. The first band represents proteinase K affected cells, second - control without proteinase K.]] |
| − | We performed whole-cell proteinase K assay based on the findings of Nicolay et al., 2012 [6] with and without bacterial cell lysis. The method that was described in the literature, should have shown us two separate bands, based on our proteins of interest domains’ size: an N‐terminal passenger domain and a C‐ terminal anchor domain (~30 and ~35 kDa in size). | + | To first characterize the protein, used for this assay (EstA), we decided to test, whether our protein is expressed onto the bacterial membrane. |
| − | + | We performed a whole-cell proteinase K assay based on the findings of Nicolay et al., 2012 [6] with and without bacterial cell lysis. The method that was described in the literature, should have shown us two separate bands, based on our proteins of interest domains’ size: an N‐terminal passenger domain and a C‐ terminal anchor domain (~30 and ~35 kDa in size). | |
| − | <br> <b>The problem</b>: this method was not accurate enough to show us whether our protein is expressed on bacterial membrane, because in SDS-PAGE imaging the protein of interest (~30 kDa) band was overlapping with the Proteinase K (~29 kDa), that was present in cells. Although different SDS-PAGE gel concentrations were used, bands still overlapped | + | |
| + | <br> <b>The problem</b>: this method was not accurate enough to show us whether our protein is expressed on bacterial membrane, because in SDS-PAGE imaging the protein of interest (~30 kDa) band was overlapping with the Proteinase K (~29 kDa), that was present in cells. Although different SDS-PAGE gel concentrations were used, bands still overlapped with each other. (Figure 1) | ||
<br> | <br> | ||
<br> | <br> | ||
| Line 34: | Line 35: | ||
====Antibody assay==== | ====Antibody assay==== | ||
| − | [[File:EstAgrakasneindukuotosgeriau.png|300px|thumb|right|Figure | + | [[File:EstAgrakasneindukuotosgeriau.png|300px|thumb|right|Figure 2: Relative fluorescence comparing induced and uninduced cells.]] |
| − | ====Quantitative data==== | + | =====Quantitative data===== |
| − | The experiment was performed using a FITC antibody, specifically designed for our system (NB600-525). | + | The experiment was performed using a FITC antibody, specifically designed for our system (''NB600-525''). Cells were co-transformed with [https://parts.igem.org/Part:BBa_K4380010 mScarlet] encoding plasmid in order to test different fluorescence in the same sample with the goal to evaluate if our proteins are expressed on the membrane and give more fluorescence than proteins inside bacterial cells. Uninduced cells were tested alongside induced and used as a control. First, we tested the cell surface display system using a microplate reader to determine if our induced cells give out the highest fluorescence signal. |
| − | <br> The first trials of proving our cell surface display system working principle showed unexpected results - <b>our uninduced cells, after several attempts, produced higher fluorescence than induced cells</b>. (Figure | + | <br> The first trials of proving our cell surface display system working principle showed unexpected results - <b>our uninduced cells, after several attempts, produced higher fluorescence than induced cells</b>. (Figure 2) |
| − | ====Qualitative data==== | + | =====Qualitative data===== |
| − | [[File:mikroskopasta.png|300px|thumb|right|Figure | + | [[File:mikroskopasta.png|300px|thumb|right|Figure 3: Fluorescence microscopy pictures comparing induced and uninduced cells.]] |
| − | After our findings have shown that our bacterial cell surface display system cannot be evaluated by antibody binding fluorescent signal quantitative result, we decided to see whether qualitative data can prove that our system is working. This time, the experiment included fluorescence microscopy | + | After our findings have shown that our bacterial cell surface display system cannot be evaluated by antibody binding fluorescent signal quantitative result, we decided to see whether qualitative data can prove that our system is working. This time, the experiment included fluorescence microscopy to visualize our cells live in real-time. This type of experiment was used to provide qualitative data over quantitative. (Figure 3) |
| − | <br> Fluorescence microscopy | + | <br> Fluorescence microscopy after incubation with antibody provided positive results - after incubation with antibody and visualization via fluorescence microscopy, we have successfully proved, that our cell surface display system works. |
Although uninduced cells emitted the most fluorescence out of all the cells, we believe this was because of <b>nonspecific antibody binding</b>. | Although uninduced cells emitted the most fluorescence out of all the cells, we believe this was because of <b>nonspecific antibody binding</b>. | ||
| − | <br>Thus, fluorescence | + | <br>Thus, quantitative fluorescence measurement was evaluated as an unuseful application to evaluate bacteria, exposing this protein to be present in the sample. |
===Directed evolution=== | ===Directed evolution=== | ||
| − | This part was specifically designed for directed evolution (DE) approaches. The genetic circuit,encoded by this part, has several distinct co-parts for | + | This part was specifically designed for directed evolution (DE) approaches. The genetic circuit, encoded by this part, has several distinct co-parts for successfully testing and performing directed evolution of the protein of interest: |
| + | <br> | ||
<br> <b> [https://parts.igem.org/Part:BBa_K4380001 ompA signaling sequence] </b> - a novel signaling sequence, used as a signaling peptide for bacterial surface display proteins to expose on bacterial surface membrane. OmpA signal peptide is a 20-residue peptide fragment of the 23-residue OmpA signal sequence cleavable by signal peptidase I. | <br> <b> [https://parts.igem.org/Part:BBa_K4380001 ompA signaling sequence] </b> - a novel signaling sequence, used as a signaling peptide for bacterial surface display proteins to expose on bacterial surface membrane. OmpA signal peptide is a 20-residue peptide fragment of the 23-residue OmpA signal sequence cleavable by signal peptidase I. | ||
<br> * <b> [https://parts.igem.org/Part:BBa_K4380006 epPCR forward] and [https://parts.igem.org/Part:BBa_K4380007 epPCR reverse] </b> - primers for amplification. | <br> * <b> [https://parts.igem.org/Part:BBa_K4380006 epPCR forward] and [https://parts.igem.org/Part:BBa_K4380007 epPCR reverse] </b> - primers for amplification. | ||
<br> * [https://parts.igem.org/Part:BBa_K4380005 17x Helix] - for protein fusion. | <br> * [https://parts.igem.org/Part:BBa_K4380005 17x Helix] - for protein fusion. | ||
<br> * [https://parts.igem.org/Part:BBa_K4380002 E-epitope] - for antibody cell surface display confirmation assay. | <br> * [https://parts.igem.org/Part:BBa_K4380002 E-epitope] - for antibody cell surface display confirmation assay. | ||
| − | <br> * [https://parts.igem.org/Part:BBa_K2694001 EstA from ''Pseudomonas aeruginosa''] - for <b> specific imobilization of target protein</b>.(Figure | + | <br> * [https://parts.igem.org/Part:BBa_K2694001 EstA from ''Pseudomonas aeruginosa''] - for <b> specific imobilization of target protein</b>. (Figure 4) |
| − | <br>Vilnius Lithuania iGEM 2022 team has successfully put two plastic binding peptides -LCI and TA2 into the same genetic circuit and | + | <br>Vilnius Lithuania iGEM 2022 team has successfully put two plastic binding peptides - LCI and TA2 into the same genetic circuit and <b>was able to get peptide libraries for specific peptide evolution approaches</b>. (Figure 5) |
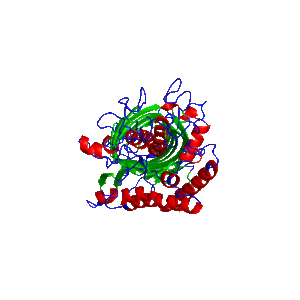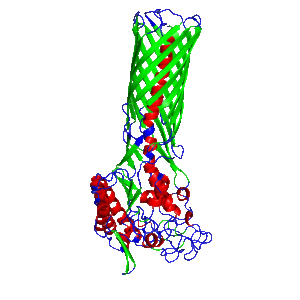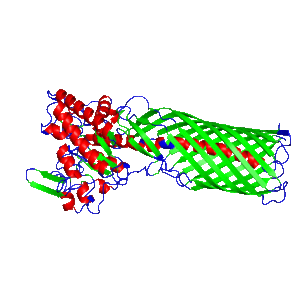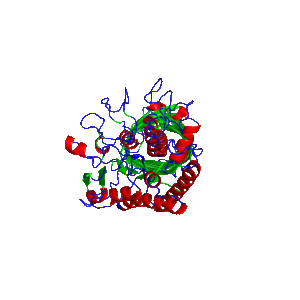
| − | [[File:EstA.gif|300px|thumb|left|Figure | + | [[File:EstA.gif|300px|thumb|left|Figure 4: 3D model of EstA protein cell surface display system. Modeled with ''Alphafold v2.1.0'']] |
| − | [[File:eppcr.png|550px|thumb|right|Figure | + | [[File:eppcr.png|550px|thumb|right|Figure 5: Peptide libraries, generated by fusing this part to two plastic-binding peptides(Parts:TA2 system:[https://parts.igem.org/Part:BBa_K4380020 BBa_K4380020] and LCI system: [https://parts.igem.org/Part:BBa_K4380019 BBa_K4380019]).]] |
| − | + | ||
| − | + | ||
| − | + | ||
<br> | <br> | ||
<br> | <br> | ||
| Line 82: | Line 81: | ||
<br> | <br> | ||
<br> | <br> | ||
| + | |||
| + | See more on the usage of this part: | ||
| + | * [https://parts.igem.org/Part:BBa_K4380019 BBa_K4380019] | ||
| + | * [https://parts.igem.org/Part:BBa_K4380020 BBa_K4380020] | ||
| + | * [https://2022.igem.wiki/vilnius-lithuania/ <b>NanoFind</b>] | ||
==References== | ==References== | ||
| − | + | [1] Becker, S., Theile, S., Heppeler, N., Michalczyk, A., Wentzel, A., Wilhelm, S., Jaeger, K.-E., & Kolmar, H. (2005). A generic system for the Escherichia coli cell-surface display of lipolytic enzymes. FEBS Letters, 579(5), 1177–1182. https://doi.org/10.1016/j.febslet.2004.12.087 | |
| − | <br>[2]Pitzler, C., Wirtz, G., Vojcic, L., Hiltl, S., Böker, A., Martinez, R., & Schwaneberg, U. (2014). A fluorescent hydrogel-based flow cytometry high-throughput screening platform for hydrolytic enzymes. Chemistry & Biology, 21(12), 1733–1742. https://doi.org/10.1016/j.chembiol.2014.10.018 | + | <br>[2] Pitzler, C., Wirtz, G., Vojcic, L., Hiltl, S., Böker, A., Martinez, R., & Schwaneberg, U. (2014). A fluorescent hydrogel-based flow cytometry high-throughput screening platform for hydrolytic enzymes. Chemistry & Biology, 21(12), 1733–1742. https://doi.org/10.1016/j.chembiol.2014.10.018 |
| − | <br>[3]Bidlingmaier, S., & Liu, B. (2015). Identification of posttranslational modification-dependent protein interactions using yeast surface-displayed human proteome libraries. Methods in Molecular Biology (Clifton, N.J.), 1319, 193–202. https://doi.org/10.1007/978-1-4939-2748-7_10 | + | <br>[3] Bidlingmaier, S., & Liu, B. (2015). Identification of posttranslational modification-dependent protein interactions using yeast surface-displayed human proteome libraries. Methods in Molecular Biology (Clifton, N.J.), 1319, 193–202. https://doi.org/10.1007/978-1-4939-2748-7_10 |
| − | <br>[4]Jose, J., & Zangen, D. (2005). Auto display of the protease inhibitor aprotinin in Escherichia coli. Biochemical and Biophysical Research Communications, 333(4), 1218–1226. https://doi.org/10.1016/j.bbrc.2005.06.028 | + | <br>[4] Jose, J., & Zangen, D. (2005). Auto display of the protease inhibitor aprotinin in Escherichia coli. Biochemical and Biophysical Research Communications, 333(4), 1218–1226. https://doi.org/10.1016/j.bbrc.2005.06.028 |
| − | <br>[5]Salema, V., & Fernández, L. Á. (2017). Escherichia coli surface display for the selection of nanobodies. Microbial Biotechnology, 10(6), 1468–1484. https://doi.org/10.1111/1751-7915.12819 | + | <br>[5] Salema, V., & Fernández, L. Á. (2017). Escherichia coli surface display for the selection of nanobodies. Microbial Biotechnology, 10(6), 1468–1484. https://doi.org/10.1111/1751-7915.12819 |
| − | <br>[6] Nicolay, T., Lemoine, L., Lievens, E., Balzarini, S., Vanderleyden, J., & Spaepen, S. (2012). Probing the applicability of autotransporter based surface display with the EstA autotransporter of Pseudomonas stutzeri A15. Microbial Cell Factories, 11, 158. https://doi.org/10.1186/1475-2859-11-158 | + | <br>[6] Nicolay, T., Lemoine, L., Lievens, E., Balzarini, S., Vanderleyden, J., & Spaepen, S. (2012). Probing the applicability of autotransporter-based surface display with the EstA autotransporter of Pseudomonas stutzeri A15. Microbial Cell Factories, 11, 158. https://doi.org/10.1186/1475-2859-11-158 |
Latest revision as of 00:45, 14 October 2022
EstA cell surface display system
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 444
Illegal BglII site found at 591
Illegal BamHI site found at 205 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 374
Illegal NgoMIV site found at 830
Illegal NgoMIV site found at 968
Illegal NgoMIV site found at 1508
Illegal NgoMIV site found at 1877 - 1000COMPATIBLE WITH RFC[1000]
Contents
Introduction
Vilnius-Lithuania Igem 2022 project NanoFind was working to create an easily accessible nanoplastic detection tool, using peptides, whose interaction with nanoplastic particles would lead to an easily interpretable response. The system itself focused on smaller protein molecules, peptides, which are modified to acquire the ability to connect to the surface of synthetic polymers – plastics. The detection system works when peptides and nanoplastic particles combine and form a sandwich complex - one nanoplastic particle is surrounded by two peptides, attached to their respective protein. The sandwich complex consisted of two main parts – one is a peptide bound to a fluorescent protein, and the other peptide is immobilized on a cellulose membrane by a cellulose binding domain.
A bacterial cell surface display system, based on the protein EstA was used as a novel way to expose the same peptides onto the bacterial membrane, allowing to amplify the signal of nanoplastic presence in a solution and finding novel peptides by using a peptide evolution protocol (PePevo). The team has generated several experimental characterizations, proving that the domain is successfully exposed on a bacterial surface, and created several new composite parts using this system.
Profile
Name: Bacterial cell surface display system, based on EstA protein from Pseudomonas aeruginosa
Origin: Synthetic, built from parts: BBa_K4380001 -> BBa_K4380006 -> BBa_K4380007 -> BBa_K4380005 -> BBa_K4380002 -> BBa_K2694001
Properties : Cell surface display protein system, which can be used as a immobilization unit on bacterial cellular membrane
Safety: Biosafety level 1 laboratory
Usage
Bacterial display systems are routinely used for the improvement of antibodies, peptides, or enzymes. One frequently used bacterial cell surface display system is based on the EstA protein. Although there are different approaches to using this protein for cell surface display exposure, one of the most frequent uses involves a mutation at active residue S38A. This mutation eliminates enzymes esterase activity but the enzyme is still able to be expressed on bacterial surfaces, therefore, can be used as an anchoring motif. The catalytically inactivated extracellular domain and the passenger domain are subsequently translocated to the cell surface [1][3] and E. coli cells can heterologously present approximately 36,000 copies of EstA on the cell surface [1][6].
Directed evolution
E. coli cells present approximately 36,000 copies of EstA on the cell surface [1]. Therefore, high presence on bacterial surfaces (especially, when recombinant proteins can be expressed in high levels), is suitable for direct evolution approaches. Direct Evolution (DE) is a method used in protein engineering that mimics the process of natural selection to steer proteins or nucleic acids toward a user-defined goal [4]. This method has successfully transformed the way we view protein engineering. During in vivo evolution, each cell is transformed with a plasmid containing a different member of the variant library. Protein EstA allows the expression of library variants on its surface, where its function can be tested. Therefore, enzymes that are presented on the cell surface or are secreted to the periplasm can successfully be engineered by directed evolution [2]. With this in mind, these systems are routinely used for the improvement of antibodies, peptides, enzymes, or nanobodies [3][4][5].
Experimental characterisation
Cell surface display prooval
Proteinase K assay
To first characterize the protein, used for this assay (EstA), we decided to test, whether our protein is expressed onto the bacterial membrane. We performed a whole-cell proteinase K assay based on the findings of Nicolay et al., 2012 [6] with and without bacterial cell lysis. The method that was described in the literature, should have shown us two separate bands, based on our proteins of interest domains’ size: an N‐terminal passenger domain and a C‐ terminal anchor domain (~30 and ~35 kDa in size).
The problem: this method was not accurate enough to show us whether our protein is expressed on bacterial membrane, because in SDS-PAGE imaging the protein of interest (~30 kDa) band was overlapping with the Proteinase K (~29 kDa), that was present in cells. Although different SDS-PAGE gel concentrations were used, bands still overlapped with each other. (Figure 1)
Antibody assay
Quantitative data
The experiment was performed using a FITC antibody, specifically designed for our system (NB600-525). Cells were co-transformed with mScarlet encoding plasmid in order to test different fluorescence in the same sample with the goal to evaluate if our proteins are expressed on the membrane and give more fluorescence than proteins inside bacterial cells. Uninduced cells were tested alongside induced and used as a control. First, we tested the cell surface display system using a microplate reader to determine if our induced cells give out the highest fluorescence signal.
The first trials of proving our cell surface display system working principle showed unexpected results - our uninduced cells, after several attempts, produced higher fluorescence than induced cells. (Figure 2)
Qualitative data
After our findings have shown that our bacterial cell surface display system cannot be evaluated by antibody binding fluorescent signal quantitative result, we decided to see whether qualitative data can prove that our system is working. This time, the experiment included fluorescence microscopy to visualize our cells live in real-time. This type of experiment was used to provide qualitative data over quantitative. (Figure 3)
Fluorescence microscopy after incubation with antibody provided positive results - after incubation with antibody and visualization via fluorescence microscopy, we have successfully proved, that our cell surface display system works.
Although uninduced cells emitted the most fluorescence out of all the cells, we believe this was because of nonspecific antibody binding.
Thus, quantitative fluorescence measurement was evaluated as an unuseful application to evaluate bacteria, exposing this protein to be present in the sample.
Directed evolution
This part was specifically designed for directed evolution (DE) approaches. The genetic circuit, encoded by this part, has several distinct co-parts for successfully testing and performing directed evolution of the protein of interest:
ompA signaling sequence - a novel signaling sequence, used as a signaling peptide for bacterial surface display proteins to expose on bacterial surface membrane. OmpA signal peptide is a 20-residue peptide fragment of the 23-residue OmpA signal sequence cleavable by signal peptidase I.
* epPCR forward and epPCR reverse - primers for amplification.
* 17x Helix - for protein fusion.
* E-epitope - for antibody cell surface display confirmation assay.
* EstA from Pseudomonas aeruginosa - for specific imobilization of target protein. (Figure 4)
Vilnius Lithuania iGEM 2022 team has successfully put two plastic binding peptides - LCI and TA2 into the same genetic circuit and was able to get peptide libraries for specific peptide evolution approaches. (Figure 5)

See more on the usage of this part:
References
[1] Becker, S., Theile, S., Heppeler, N., Michalczyk, A., Wentzel, A., Wilhelm, S., Jaeger, K.-E., & Kolmar, H. (2005). A generic system for the Escherichia coli cell-surface display of lipolytic enzymes. FEBS Letters, 579(5), 1177–1182. https://doi.org/10.1016/j.febslet.2004.12.087
[2] Pitzler, C., Wirtz, G., Vojcic, L., Hiltl, S., Böker, A., Martinez, R., & Schwaneberg, U. (2014). A fluorescent hydrogel-based flow cytometry high-throughput screening platform for hydrolytic enzymes. Chemistry & Biology, 21(12), 1733–1742. https://doi.org/10.1016/j.chembiol.2014.10.018
[3] Bidlingmaier, S., & Liu, B. (2015). Identification of posttranslational modification-dependent protein interactions using yeast surface-displayed human proteome libraries. Methods in Molecular Biology (Clifton, N.J.), 1319, 193–202. https://doi.org/10.1007/978-1-4939-2748-7_10
[4] Jose, J., & Zangen, D. (2005). Auto display of the protease inhibitor aprotinin in Escherichia coli. Biochemical and Biophysical Research Communications, 333(4), 1218–1226. https://doi.org/10.1016/j.bbrc.2005.06.028
[5] Salema, V., & Fernández, L. Á. (2017). Escherichia coli surface display for the selection of nanobodies. Microbial Biotechnology, 10(6), 1468–1484. https://doi.org/10.1111/1751-7915.12819
[6] Nicolay, T., Lemoine, L., Lievens, E., Balzarini, S., Vanderleyden, J., & Spaepen, S. (2012). Probing the applicability of autotransporter-based surface display with the EstA autotransporter of Pseudomonas stutzeri A15. Microbial Cell Factories, 11, 158. https://doi.org/10.1186/1475-2859-11-158