Difference between revisions of "Part:BBa K4273008"
| Line 7: | Line 7: | ||
encoding ATP-grasp ligase(AG-L) that converts 4-deoxygadusol (4-DG) to mycosporine-glycine(MG). | encoding ATP-grasp ligase(AG-L) that converts 4-deoxygadusol (4-DG) to mycosporine-glycine(MG). | ||
| − | + | ==Usage in Biology== | |
| − | === | + | Np5598 is a gene found in cyanobacteria Nostoc punctiforme that encodes for AGL whereas NlmysD encodes for AlaL in Nostoc linckia. AGL-AlaL allows 4-DG to be converted into the MAAs shinorine or porphyra-334. In our experiment, we found that AlaL encoded by NlmysD has a strong preference toward the amino acid threonine. Therefore, this part could efficiently produce the MAA porphyra-334 primarily, making it the first successful case of producing pure samples of porphyra-334. This part is within our part collection that allows efficiently production of MAAs in S. cerevisiae. |
| + | Our part collection contains necessary genes to produce gadusol and the MAAs shinorine, porphyra-334, and palythine at a high rate. Xyl1, Xyl2, and Xyl3 are genes that allow S. cerevisiae to utilize xylose to produce S7P. DDGS and OMT converts S7P to the precursor of MAA, 4-deoxygadusol (4-DG). AGL converts 4-DG into M-glycine (MG). AlaL, by adding either serine or threonine, produces shinorine and porphyra-334, respectively. MysH could be added to the circuit of shinorine to produce palythine. S7P could also be converted into gadusol under the catalyzation of EEVS and M-Tox. In this part collection, we included multiple pathways and methods to increase the production of the upstream S7P and downstream MAAs. | ||
| + | This part collection can provide inspiration and efficient methods to utilize the penta phosphate pathway or to produce other types of MAAs in S. cerevisiae for other teams. | ||
| + | |||
| + | We selected promoters pTDH3 and pPGK1 due to their stability expression in S. cerevisiae (Apel et. al., 2016). These promoters are shown to have stable and strong expression in YPD culture mediums. Among the three, pTDH3 has highest stability and strength, followed by pPGK1. For expression of AGL and AlaL enzymes, we used pTDH3 for AGL and pPGK1 for AlaL. In order to optimize our production, we inserted this part into the yeast’s genome at position 106, chromosome I (Apel et. al., 2016). | ||
| + | |||
| + | |||
| + | ==Experiment== | ||
| + | [Design and Absorbance of Nine AGL-AlaL Combinations] | ||
| + | We expressed AGL-AlaL genes first using plasmid vector and then through genome insertion. Due to the existence of multiple different types of AGL and AlaL with different efficiency and amino acid preference in nature, we selected ligases from three different marine organisms: Nostoc punctiform (Np5598 and Np5597), Nostoc linckia (NlmysC and NImysD) and Actinosynnema mirum (Am4257 and Am4256), and expected to create nine combinations of AGL-AlaL. We used Lee's yeast toolkit to produce Level1 plasmids containing only AGL or AlaL, and used Golden Gate assembly on the basis of Level1 to construct Level2 plasmid containing the 9 combinations of AGL and AlaL. Finally, we transformed these Level2 plasmids into SC.L3 strains, which are S. cerevisiae which could produce 4-DG efficiently from the addition of DDGS and OMT, to form strain SC.L5. | ||
| + | |||
| + | [[Image:t-links-china-figure1.png|thumb|left|900px|'''Figure 1: Different combinations of AGL-ALAL genes found from different MAA-producing marine organisms. We used the strongest yeast constitutive promoter pTDH3 to express all AG-L genes, and the strong promoter pPGK1 to express all ALA-L genes, then inserted the 9 different combinations into 2μ plasmid vectors and transformed the plasmids into L3 to obtain L5 strain''']] | ||
| + | |||
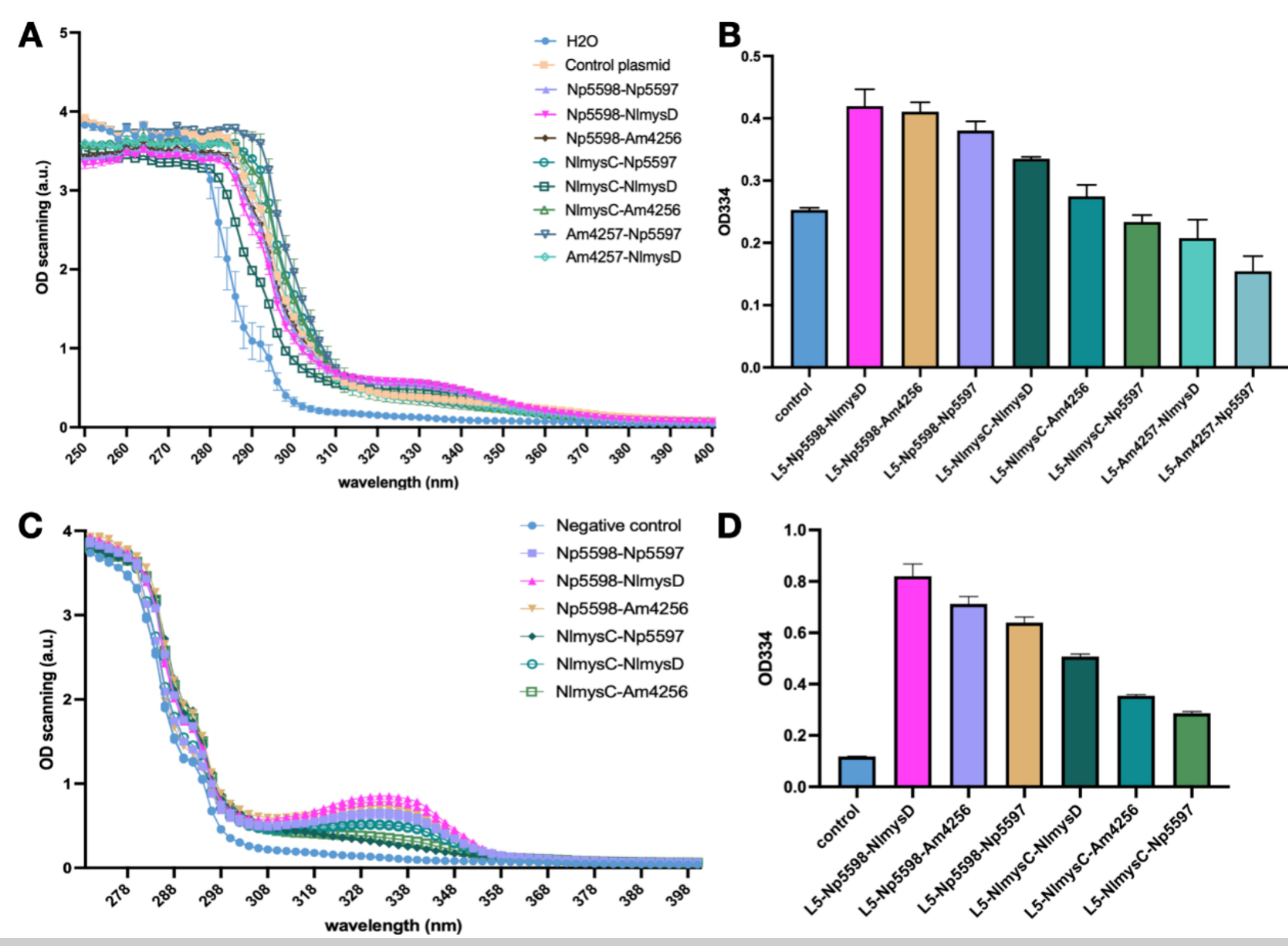
| + | <br>Shinorine and porphyra-334 has similar absorption curves with an absorption peak at 334 nm. We cultivated the SC.L5 yeast using a SC-Ura culture medium with 1% glucose and 1% xylose and tested the absorption spectrum of the supernatant of the fermentation broth. Other than the Am4257-Am4256 combination yeast which failed to grow, there is an obvious absorption peak of 334 nm for the other 8 combinations. We found that the absorption peak of AGL Np5598 series was significantly higher than that of NlmysC and Am4257. In ALAL series, NlmysD has the highest absorption peak, followed by Am4256 and Np5597. We selected the 6 samples with higher peak and lysed them to preform OD scanning on the lysate. The scanning results after lysis further performed that Np5598 is the most efficient AGL and NlmysD is the most efficient AlaL. We think we have found an optimal combination of these two enzymes and have successfully produced MAAs. | ||
| + | |||
| + | [[Image:t-links-china-figure2.png|thumb|left|900px|'''Figure 2: Absorption spectrum of the AGL-AlaL combinations of L5 strains that produces shinorine or porphyra-334. We scanned the OD of broth supernatant after 72 hours of fermentation of 8 groups of yeast (except for Am4257-Am4256 which failed to grow), which shows a clear absorption peak at 334nm (A). We compared of OD334 values of the samples, discovering that Np5598 seems to be the most efficient AGL, while NlmysD being the most efficient AlaL. We conducted the same experiment with the yeast lysate of the 6 most efficient combinations and found an even more significant absorption peak (C). The OD334 value of the lysate supernatant affirms our previous conclusion that Np5598 is the best AGL and NlmysD the best AlaL (D).''']] | ||
| + | |||
| + | <br>Determining the Amino Acid Preference of Np5597 and NlmysD | ||
| + | Moreover, because AlaL has a preference for different amino acids to produce shinorine and porphyra-334, we need to further confirm the type of MAA produced by different strains. To do so, we used High-performing Liquid Chromatology (HPLC) and Mass Spectrometer (MS) technology. Due to the lack of standard samples of these MAAs in the market, we extracted MAAs from nori samples using methods we learnt through our human practice activities and used the extractives as standard. We found that the metabolite of Np5598-NlmysD mainly has the absorption spectrum of porphyra-334 according to HPLC, and the result of MS also proved that mainly porphyra-334 exist in the metabolite (m/q = 347). This shows that NlmysD has a strong selective preference toward the amino acid Threonine, and thus will mainly produce porphyra-334 if both Threonine and Serine are present in the environment. This is the first successful case of producing mainly 334 only. Np5597, on the other hand, shows shinorine's absorption peak, meaning it has preference toward serine. Therefore, we decided to use Np5598-NlmysD for porphyra-334 production and Np5598-NlmysD for shinorine production. | ||
| + | |||
| + | [[Image:t-links-china-figure3.png|thumb|left|900px|'''Figure 3: High Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) test of Np5598-NlmysD and Np5598-Np5597 compared with nori samples. Shinorine and Porphyra-334 lack a standard on the market, thus we decided to extract pure MAA from nori (Porphyra spp.) to use as standard. HPLC results (A) and MS results (B) show a peak in shinorine and porphyra-334, which proves that there is high concentration in nori extractives, making it a reliable standard. In Np5598-NlmysD’s fermentation broth, HPLC results (C) and MS results (D) show that mostly porphyra-334 exists. However, in Np5598-Np5597’s liquid, HPLC (E) and MS (F) results show mostly shinorine.''']] | ||
| + | |||
| + | <br>Optimization Through Genome Insertion | ||
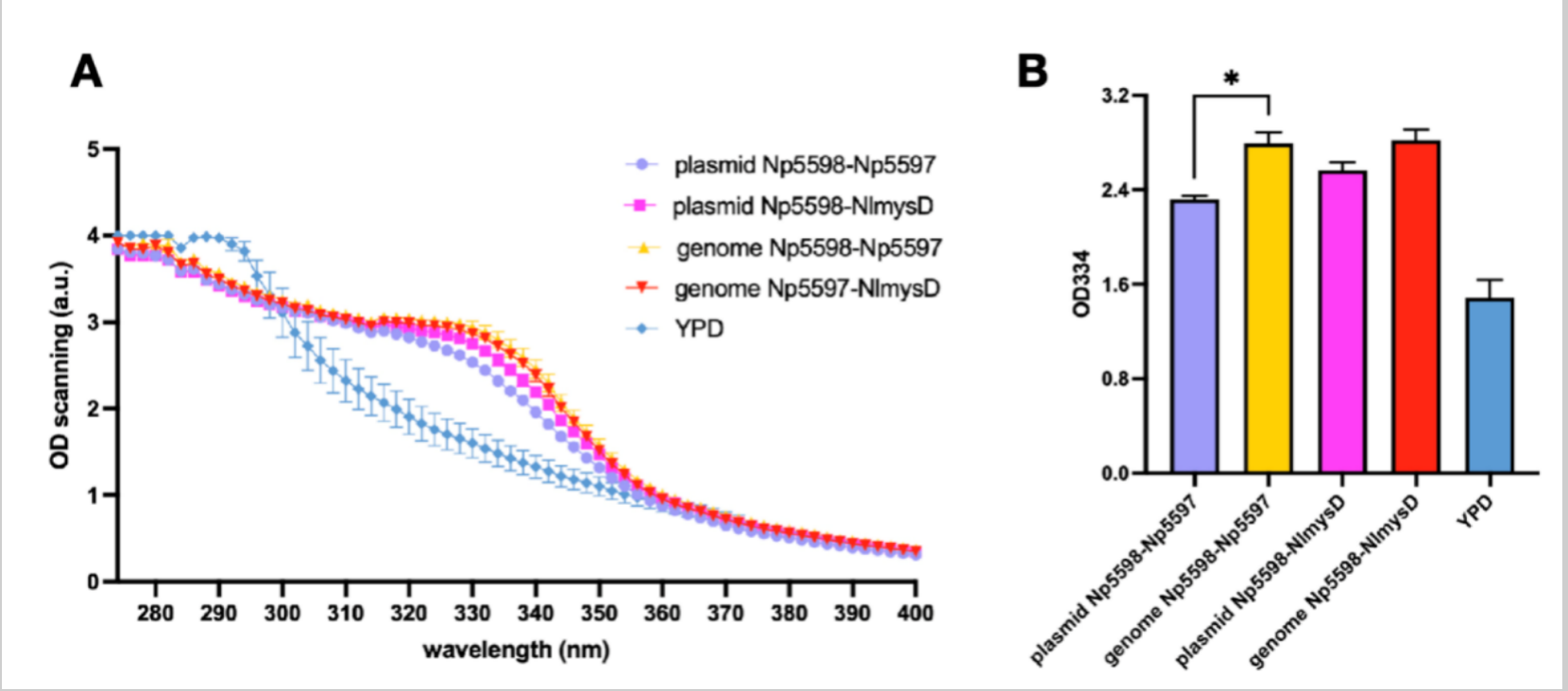
| + | Considering the instability and lower efficiency of using plasmid expresssion, we decided to insert Np5598-NlmysD and Np5598-Np5597, the most efficient combinations to produce porphyra-334 and shinorine respectively (Figure 9 & 10) into SC.L6's genome at chromosome I, position 106 (SC.L6 is SC.L5 with OMT-DDGS inserted at Nqm1 in order to increase MAAs production). We named the new strains SC.L8 series. After fermentation for 72 hours in YPD medium, we compared the OD 334 absorption of the broth's supernatant between SC.L7 series, SC.L6 with the plasmids pYT-pTDH3-Np5598-tTDH1-pPGK1-AlaL-tPGK1 (AlaL includes Np5597 and NlmysD) and SC.L8 series. We found that the OD value of L8 series (for both Np5598-NlmysD and Np5598-Np5597) with genome recombinant, showed a steady increase of approximately 20% compared with that of L7 series with plasmid expression. Finally, the nori extract with a higher purity of shinorine and porphyra-334 is used as the standard sample to obtain rough yield data of SC.L8 series. Porphyra-334’s yield reached 270.7mg/L in L8-Np5598-NlmysD. | ||
| + | |||
| + | |||
| + | [[Image:t-links-china-figure4.png|thumb|left|900px|'''Figure 4: Genome insertion of Np5598-NlmysD and Np5598-Np5597. To further increase and stabilize Shinorine and Porphyra-334 production, we inserted the genes Np5598-NlmysD and Np5598-Np5597 into the yeast’s genome at position 106 of chromosome I (Amanda R. et.al. 2017). After OD scanning, we found that the absorption peak of after genome insertion is higher than plasmid vector (A). We compared the OD334 value of plasmid transformation and genome insertion and found that there is about 20% increase in genome insertion expression.''']] | ||
<!-- --> | <!-- --> | ||
Revision as of 14:24, 12 October 2022
NpR5598
The third gene of gene clusters involved in biosynthesis of shinorine in cyanobacteria N. punctiforme.
encoding ATP-grasp ligase(AG-L) that converts 4-deoxygadusol (4-DG) to mycosporine-glycine(MG).
Usage in Biology
Np5598 is a gene found in cyanobacteria Nostoc punctiforme that encodes for AGL whereas NlmysD encodes for AlaL in Nostoc linckia. AGL-AlaL allows 4-DG to be converted into the MAAs shinorine or porphyra-334. In our experiment, we found that AlaL encoded by NlmysD has a strong preference toward the amino acid threonine. Therefore, this part could efficiently produce the MAA porphyra-334 primarily, making it the first successful case of producing pure samples of porphyra-334. This part is within our part collection that allows efficiently production of MAAs in S. cerevisiae. Our part collection contains necessary genes to produce gadusol and the MAAs shinorine, porphyra-334, and palythine at a high rate. Xyl1, Xyl2, and Xyl3 are genes that allow S. cerevisiae to utilize xylose to produce S7P. DDGS and OMT converts S7P to the precursor of MAA, 4-deoxygadusol (4-DG). AGL converts 4-DG into M-glycine (MG). AlaL, by adding either serine or threonine, produces shinorine and porphyra-334, respectively. MysH could be added to the circuit of shinorine to produce palythine. S7P could also be converted into gadusol under the catalyzation of EEVS and M-Tox. In this part collection, we included multiple pathways and methods to increase the production of the upstream S7P and downstream MAAs. This part collection can provide inspiration and efficient methods to utilize the penta phosphate pathway or to produce other types of MAAs in S. cerevisiae for other teams.
We selected promoters pTDH3 and pPGK1 due to their stability expression in S. cerevisiae (Apel et. al., 2016). These promoters are shown to have stable and strong expression in YPD culture mediums. Among the three, pTDH3 has highest stability and strength, followed by pPGK1. For expression of AGL and AlaL enzymes, we used pTDH3 for AGL and pPGK1 for AlaL. In order to optimize our production, we inserted this part into the yeast’s genome at position 106, chromosome I (Apel et. al., 2016).
Experiment
[Design and Absorbance of Nine AGL-AlaL Combinations] We expressed AGL-AlaL genes first using plasmid vector and then through genome insertion. Due to the existence of multiple different types of AGL and AlaL with different efficiency and amino acid preference in nature, we selected ligases from three different marine organisms: Nostoc punctiform (Np5598 and Np5597), Nostoc linckia (NlmysC and NImysD) and Actinosynnema mirum (Am4257 and Am4256), and expected to create nine combinations of AGL-AlaL. We used Lee's yeast toolkit to produce Level1 plasmids containing only AGL or AlaL, and used Golden Gate assembly on the basis of Level1 to construct Level2 plasmid containing the 9 combinations of AGL and AlaL. Finally, we transformed these Level2 plasmids into SC.L3 strains, which are S. cerevisiae which could produce 4-DG efficiently from the addition of DDGS and OMT, to form strain SC.L5.

Shinorine and porphyra-334 has similar absorption curves with an absorption peak at 334 nm. We cultivated the SC.L5 yeast using a SC-Ura culture medium with 1% glucose and 1% xylose and tested the absorption spectrum of the supernatant of the fermentation broth. Other than the Am4257-Am4256 combination yeast which failed to grow, there is an obvious absorption peak of 334 nm for the other 8 combinations. We found that the absorption peak of AGL Np5598 series was significantly higher than that of NlmysC and Am4257. In ALAL series, NlmysD has the highest absorption peak, followed by Am4256 and Np5597. We selected the 6 samples with higher peak and lysed them to preform OD scanning on the lysate. The scanning results after lysis further performed that Np5598 is the most efficient AGL and NlmysD is the most efficient AlaL. We think we have found an optimal combination of these two enzymes and have successfully produced MAAs.
Determining the Amino Acid Preference of Np5597 and NlmysD
Moreover, because AlaL has a preference for different amino acids to produce shinorine and porphyra-334, we need to further confirm the type of MAA produced by different strains. To do so, we used High-performing Liquid Chromatology (HPLC) and Mass Spectrometer (MS) technology. Due to the lack of standard samples of these MAAs in the market, we extracted MAAs from nori samples using methods we learnt through our human practice activities and used the extractives as standard. We found that the metabolite of Np5598-NlmysD mainly has the absorption spectrum of porphyra-334 according to HPLC, and the result of MS also proved that mainly porphyra-334 exist in the metabolite (m/q = 347). This shows that NlmysD has a strong selective preference toward the amino acid Threonine, and thus will mainly produce porphyra-334 if both Threonine and Serine are present in the environment. This is the first successful case of producing mainly 334 only. Np5597, on the other hand, shows shinorine's absorption peak, meaning it has preference toward serine. Therefore, we decided to use Np5598-NlmysD for porphyra-334 production and Np5598-NlmysD for shinorine production.

Optimization Through Genome Insertion
Considering the instability and lower efficiency of using plasmid expresssion, we decided to insert Np5598-NlmysD and Np5598-Np5597, the most efficient combinations to produce porphyra-334 and shinorine respectively (Figure 9 & 10) into SC.L6's genome at chromosome I, position 106 (SC.L6 is SC.L5 with OMT-DDGS inserted at Nqm1 in order to increase MAAs production). We named the new strains SC.L8 series. After fermentation for 72 hours in YPD medium, we compared the OD 334 absorption of the broth's supernatant between SC.L7 series, SC.L6 with the plasmids pYT-pTDH3-Np5598-tTDH1-pPGK1-AlaL-tPGK1 (AlaL includes Np5597 and NlmysD) and SC.L8 series. We found that the OD value of L8 series (for both Np5598-NlmysD and Np5598-Np5597) with genome recombinant, showed a steady increase of approximately 20% compared with that of L7 series with plasmid expression. Finally, the nori extract with a higher purity of shinorine and porphyra-334 is used as the standard sample to obtain rough yield data of SC.L8 series. Porphyra-334’s yield reached 270.7mg/L in L8-Np5598-NlmysD.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
