Difference between revisions of "Part:BBa K4274010"
| (5 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K4274010 short</partinfo> | <partinfo>BBa_K4274010 short</partinfo> | ||
| − | degQ gene is pleiotropic regulator gene encoding a 46 amino acid polypeptide in <i>Bacillus subtilis</i> 168. It can regulate the expression of a variety of exocrine enzymes and the production of antibacterial substances. Previous studies have shown that the importance of degQ gene in | + | degQ gene is pleiotropic regulator gene encoding a 46 amino acid polypeptide in <i>Bacillus subtilis</i> 168. It can regulate the expression of a variety of exocrine enzymes and the production of antibacterial substances. Previous studies have shown that the importance of degQ gene in fengycin‘s production. Therefore, degQ gene was knocked-in <i>Bacillus subtilis</i> 168 together with sfp gene from <i>Bacillus amyloliquefaciens</i> FZB42 (Part: BBa_K4274009). |
==Usage and Biology== | ==Usage and Biology== | ||
| − | degQ gene (Gene ID: 936547) is pleiotropic regulator gene encoding a 46 amino acid polypeptide in Bacillus subtilis 168. It can regulate the expression of a variety of exocrine enzymes and the production of antibacterial substances. It is reported that increased expression of degQ in B. subtilis 168 results in a 7-10 fold increase in antibiotic production. It is because a T base at position -10 in the promoter region of degQ is mutated into a C, resulting in the inability to express degQ. | + | degQ gene (Gene ID: 936547) is pleiotropic regulator gene encoding a 46 amino acid polypeptide in <i>Bacillus subtilis</i> 168. It can regulate the expression of a variety of exocrine enzymes and the production of antibacterial substances. It is reported that increased expression of degQ in <i>B. subtilis</i> 168 results in a 7-10 fold increase in antibiotic production. It is because a T base at position -10 in the promoter region of degQ is mutated into a C, resulting in the inability to express degQ. |
| − | In this case, DNA sequence of degQ gene under the regulation of p43 promoter was knocked-in the region of the natural sfp gene of B. subtilis. It was used in the composite part PvanP*-sfp_target-sfp_HA_US-p43-K4274013-sfp-K4274014-degQ-sfp_HA_DS (Part: BBa_K4274035) to realize fengycins’ production in B. subtilis. This can be used for other teams working on fengycin production or research on the antibiotic properties of cyclolipopeptides. | + | |
| + | <br>In this case, DNA sequence of degQ gene under the regulation of p43 promoter was knocked-in the region of the natural sfp gene of <i>B. subtilis</i>. It was used in the composite part PvanP*-sfp_target-sfp_HA_US-p43-K4274013-sfp-K4274014-degQ-sfp_HA_DS (Part: BBa_K4274035) to realize fengycins’ production in <i>B. subtilis</i>. This can be used for other teams working on fengycin production or research on the antibiotic properties of cyclolipopeptides. | ||
| + | |||
| + | ==Characterization== | ||
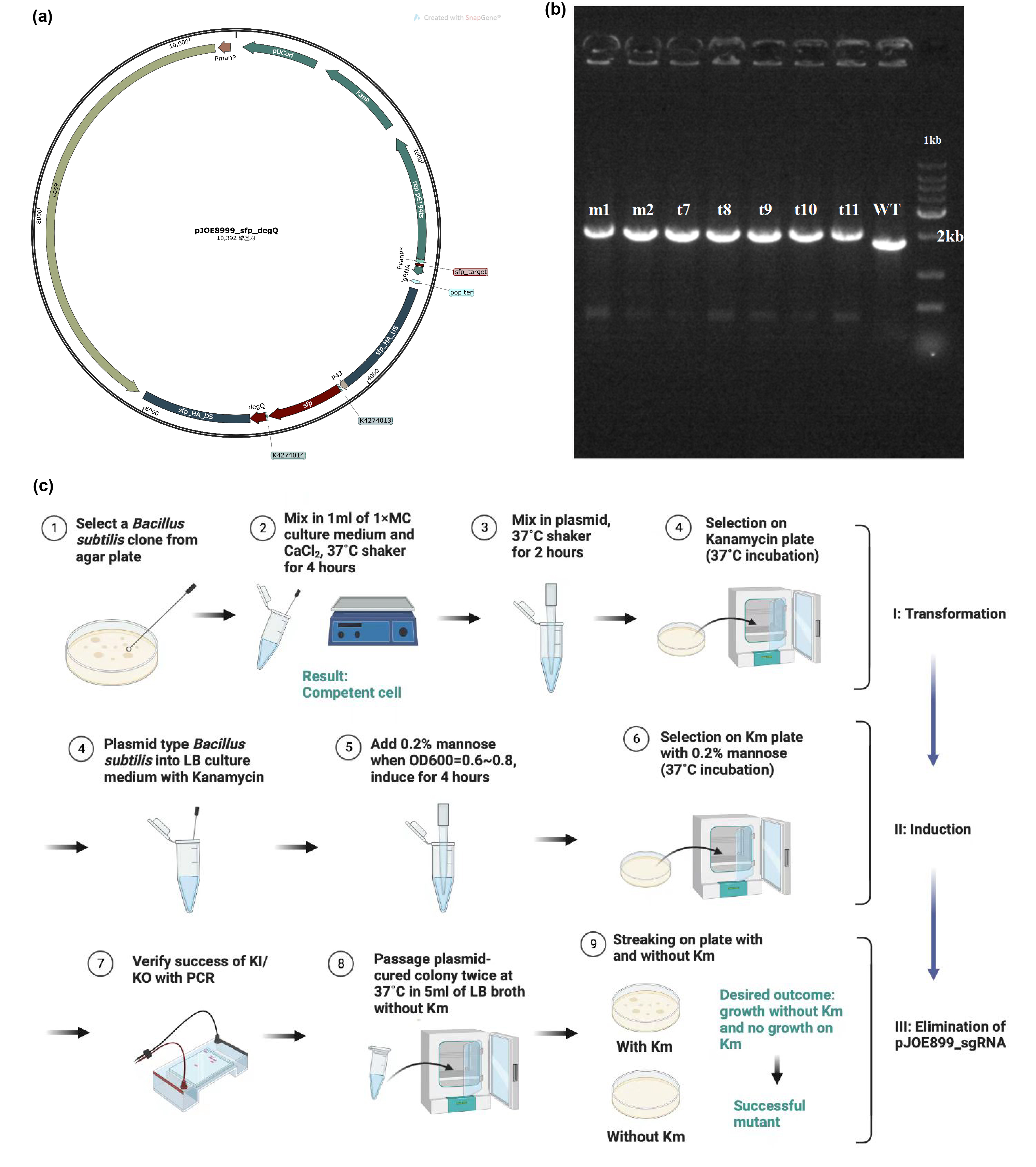
| + | The main composite of our product, fengycins, is produced by engineering <i>Bacillus subtilis</i> 168. As it is shown in the demonstration in description, sfp and degQ genes are critical to the biosynthesis of fengycins. Besides, <i>Bacillus subtilis</i> 168 possesses a natural but invalid sfp gene, and the functional degQ gene is silenced with the regulation of a weak promoter.Thus, with the help of pJOE8999_sfp_degQ plasmid (Figure 1a), we knocked in both sfp gene and degQ gene in the region of the natural sfp gene of <i>Bacillus subtilis</i> 168 after being knocking out. Under the guidance of protocol, we successfully transformed and induced the plasmid to function as a gene editor (Figure 1c). After induction, 7 strains were selected for PCR and electrophoresis verification. Since successful knock-in would would result in a 252 bps increase in the genome of <i>Bacillus subtilis</i> 168, the electrophoresis result conveys preliminarily that all of these strains has achieved our target of constructing the fengycins producers (Figure 1b). | ||
| + | [[Image:Parts-keystone-fengycin1.jpeg|thumbnail|750px|center|'''Figure 1:''' | ||
| + | Edting the genome of <i>Bacillus subtilis</i> 168 to enable it to produce fengycins. (a) Plasmid design for knocking out the invalid sfp gene of <i>Bacillus subtilis</i> 168 and knocking in the sfp gene from B. amyloliquefaciens FZB42 and degQ gene from <i>Bacillus subtilis</i> 168. (b) Electrophoresis results show that gene editing is successful. (c) Protocol about transformation, induction and elimination of pJOE8999 plasmid in <i>Bacillus subtilis</i> 168. ]] | ||
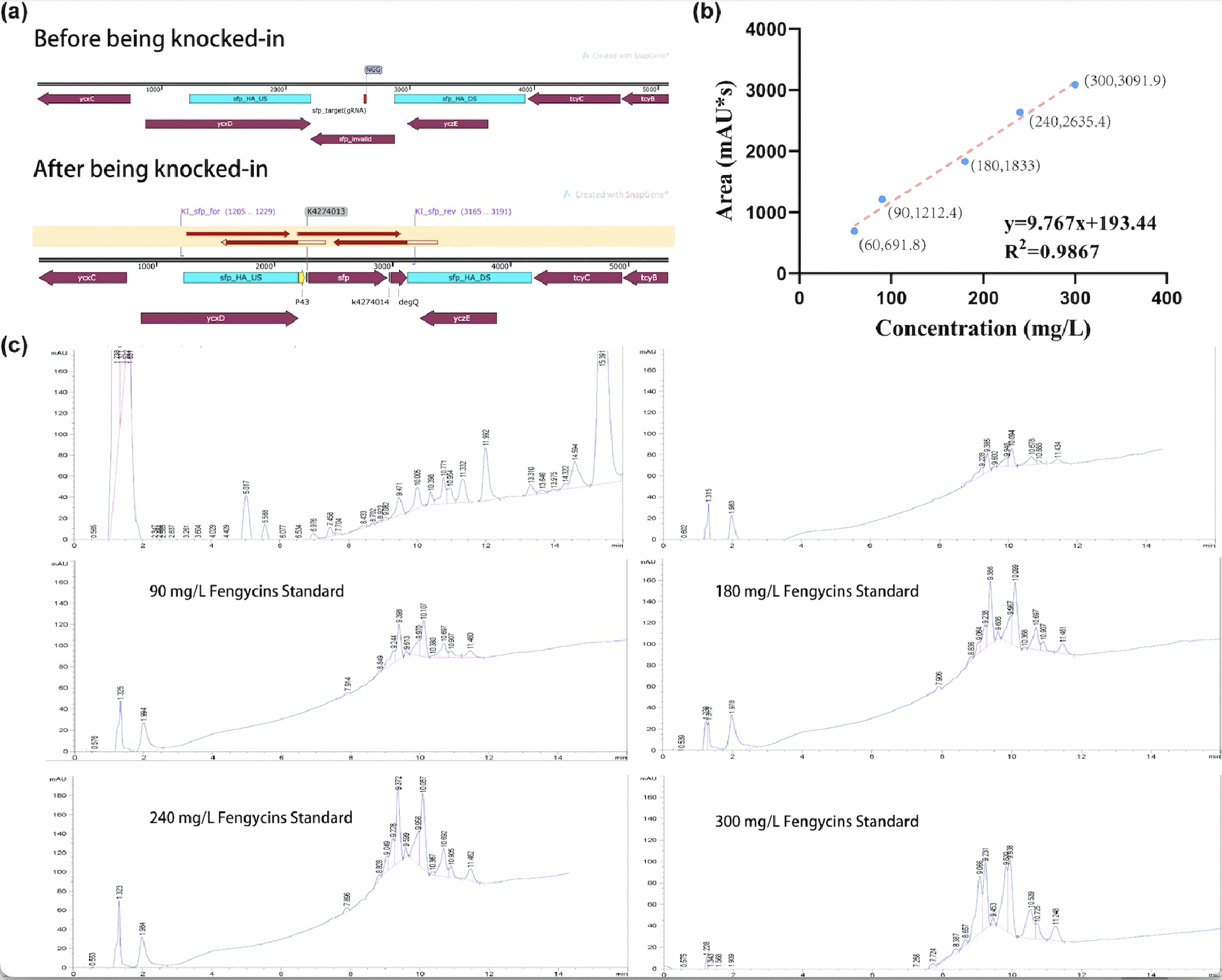
| + | After sequencing further verified that the successful construction of the fengycins producing strain, we used the strain for fermentation (Figure 2a). For the quantification of fengycins production, the methanol - extract obtained from the fermentation broth was analyzed by high performance liquid chromatography (HPLC). The peak of fengycins appeared at the retention time of 8-11.5 min under methanol mobile phase (Figure 2c). The injection sample was a solution of lipopeptide extract from 30 mL of fermentation broth dissolved in 7 mL methanol. Besides, various concentrations of fengycins standard is also injected for the construction of the standard curve of peak area obtained by HPLC (y) and its corresponding fengycins’ concentration (x). Based on the standard curve, we got the regression curve and its corresponding formula y=9.767x+193.44, and R2>0.98 displays that the regression equation fits the observed values (Figure 2b). Substituting y=1242.3 obtained from the sample into the regression equation, the concentration of fengycin in methanol solution can be calculated as 107.4 mg/L, that is, 25.06 mg/L of fengycins can be achieved by shaking-flask fermentation. | ||
| + | [[Image:Parts-keystone-fengycin2-1.jpeg|750px|thumbnail|center|'''Figure 2:''' | ||
| + | Quantification analysis of fengycins production. (a) The sequencing result of engineering <i>Bacillus subtilis</i> 168 strain for production. (b) The standard curve of peak area obtained by HPLC (y) and its corresponding fengycins' concentration (x). (c) HPLC results of sample and various concentrations of fengycins standard. ]] | ||
==Source== | ==Source== | ||
| − | <i> | + | <i>Bacillus subtilis</i> 168 |
==Sequence and Features== | ==Sequence and Features== | ||
| − | <partinfo> | + | <partinfo>BBa_K4274010 SequenceAndFeatures</partinfo> |
==References== | ==References== | ||
| − | [1] | + | [1]Tsuge K., Ano T., Hirai M., et al. The Genes degQ, pps, and Ipa-8(sfp) Are Responsible for Conversion of Bacillus subtilis 168 to Plipastin Production. Antimicrobial Agents and Chemo. 43(9), 2183-2192 (1999). https://doi.org/10.1128/AAC.43.9.2183. |
| − | [2] | + | [2]Gao L., Han J., Liu H., et al. Plipastin and surfactin coproduction by Bacillus subtilis pB2-L and their effects on microorganisms. Antonie van Leeuwenhoek. 110 (3), 1007-1018 (2017). https://doi.org/10.1007/s10482-017-0874-y |
| − | [3] | + | [3]Yin Y., Tan W., Wen J., et al. Increasing fengycin production by strengthening the fatty acid synthesis pathway and optimizing fermentation conditions. Biochem. Engineering Journal. 177 (21), 1082 (2021). https://doi.org/10.1016/j.bej.2021.108235 |
Latest revision as of 12:48, 12 October 2022
degQ
degQ gene is pleiotropic regulator gene encoding a 46 amino acid polypeptide in Bacillus subtilis 168. It can regulate the expression of a variety of exocrine enzymes and the production of antibacterial substances. Previous studies have shown that the importance of degQ gene in fengycin‘s production. Therefore, degQ gene was knocked-in Bacillus subtilis 168 together with sfp gene from Bacillus amyloliquefaciens FZB42 (Part: BBa_K4274009).
Usage and Biology
degQ gene (Gene ID: 936547) is pleiotropic regulator gene encoding a 46 amino acid polypeptide in Bacillus subtilis 168. It can regulate the expression of a variety of exocrine enzymes and the production of antibacterial substances. It is reported that increased expression of degQ in B. subtilis 168 results in a 7-10 fold increase in antibiotic production. It is because a T base at position -10 in the promoter region of degQ is mutated into a C, resulting in the inability to express degQ.
In this case, DNA sequence of degQ gene under the regulation of p43 promoter was knocked-in the region of the natural sfp gene of B. subtilis. It was used in the composite part PvanP*-sfp_target-sfp_HA_US-p43-K4274013-sfp-K4274014-degQ-sfp_HA_DS (Part: BBa_K4274035) to realize fengycins’ production in B. subtilis. This can be used for other teams working on fengycin production or research on the antibiotic properties of cyclolipopeptides.
Characterization
The main composite of our product, fengycins, is produced by engineering Bacillus subtilis 168. As it is shown in the demonstration in description, sfp and degQ genes are critical to the biosynthesis of fengycins. Besides, Bacillus subtilis 168 possesses a natural but invalid sfp gene, and the functional degQ gene is silenced with the regulation of a weak promoter.Thus, with the help of pJOE8999_sfp_degQ plasmid (Figure 1a), we knocked in both sfp gene and degQ gene in the region of the natural sfp gene of Bacillus subtilis 168 after being knocking out. Under the guidance of protocol, we successfully transformed and induced the plasmid to function as a gene editor (Figure 1c). After induction, 7 strains were selected for PCR and electrophoresis verification. Since successful knock-in would would result in a 252 bps increase in the genome of Bacillus subtilis 168, the electrophoresis result conveys preliminarily that all of these strains has achieved our target of constructing the fengycins producers (Figure 1b).
After sequencing further verified that the successful construction of the fengycins producing strain, we used the strain for fermentation (Figure 2a). For the quantification of fengycins production, the methanol - extract obtained from the fermentation broth was analyzed by high performance liquid chromatography (HPLC). The peak of fengycins appeared at the retention time of 8-11.5 min under methanol mobile phase (Figure 2c). The injection sample was a solution of lipopeptide extract from 30 mL of fermentation broth dissolved in 7 mL methanol. Besides, various concentrations of fengycins standard is also injected for the construction of the standard curve of peak area obtained by HPLC (y) and its corresponding fengycins’ concentration (x). Based on the standard curve, we got the regression curve and its corresponding formula y=9.767x+193.44, and R2>0.98 displays that the regression equation fits the observed values (Figure 2b). Substituting y=1242.3 obtained from the sample into the regression equation, the concentration of fengycin in methanol solution can be calculated as 107.4 mg/L, that is, 25.06 mg/L of fengycins can be achieved by shaking-flask fermentation.
Source
Bacillus subtilis 168
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
[1]Tsuge K., Ano T., Hirai M., et al. The Genes degQ, pps, and Ipa-8(sfp) Are Responsible for Conversion of Bacillus subtilis 168 to Plipastin Production. Antimicrobial Agents and Chemo. 43(9), 2183-2192 (1999). https://doi.org/10.1128/AAC.43.9.2183.
[2]Gao L., Han J., Liu H., et al. Plipastin and surfactin coproduction by Bacillus subtilis pB2-L and their effects on microorganisms. Antonie van Leeuwenhoek. 110 (3), 1007-1018 (2017). https://doi.org/10.1007/s10482-017-0874-y
[3]Yin Y., Tan W., Wen J., et al. Increasing fengycin production by strengthening the fatty acid synthesis pathway and optimizing fermentation conditions. Biochem. Engineering Journal. 177 (21), 1082 (2021). https://doi.org/10.1016/j.bej.2021.108235
