Difference between revisions of "Part:BBa K4140008"
Ahmed Wael (Talk | contribs) (→Improvement by Directed Evolution) |
Ahmed Mattar (Talk | contribs) (→Experimental validation of the improvement) |
||
| (48 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
==Usage== | ==Usage== | ||
| − | alpha domain of ß-galactosidase (our reporter protein) which is produced in response to high levels of phenylalanine and generates a blue product when X-gal is cleaved which is a soluble colorless molecule that is a substrate of ß-galactosidase and generates a blue product when cleaved, is used to detect the alpha-complementation | + | alpha domain of ß-galactosidase (our reporter protein) which is produced in response to high levels of phenylalanine and generates a blue product when X-gal is cleaved which is a soluble colorless molecule that is a substrate of ß-galactosidase and generates a blue product when cleaved, is used to detect the alpha-complementation. We used beta-galactosidase enzyme to turn the color into blue once increased level of phenylalanine is detected via exported extracellularly via tagging by kp-sp to bin with its substrate (X-gal). |
| + | ===Improvements=== | ||
| + | We improved the part <html><a href="https://parts.igem.org/Part:BBa_E0033">BBa_E0033</a></html> by :- | ||
| − | |||
=== Improvement by KP-SP tagging === | === Improvement by KP-SP tagging === | ||
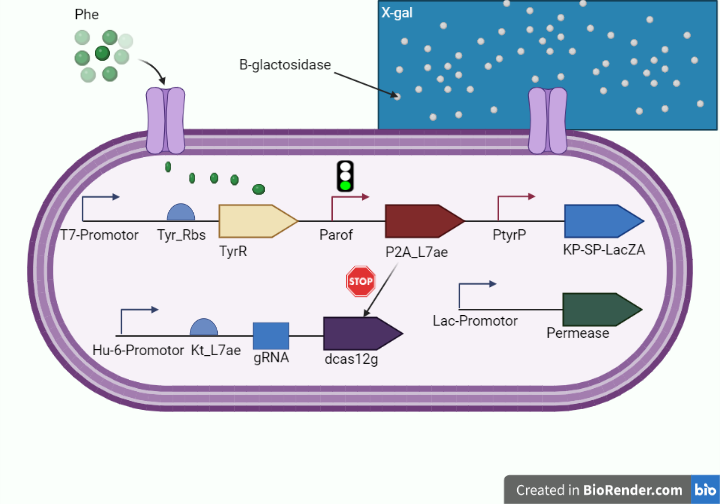
| − | This year, we enhanced the LacZ alpha gene by incorporating a peptide signal that controls the secretion of B galactosidase extracellularly in an effort to improve the cleavage capacity of the X gal, heighten the intensity of the dark blue color emitted by the X gal product, and reduce the amount of time required to receive the results after performing the test on the chip | + | This year, we enhanced the LacZ alpha gene by incorporating a peptide signal that controls the secretion of B galactosidase extracellularly (KP-SP tagging) in an effort to improve the cleavage capacity of the X gal, heighten the intensity of the dark blue color emitted by the X gal product, and reduce the amount of time required to receive the results after performing the test on the chip. as shown in figure 1 and figure 2. |
| − | Improvement of LacZ alpha gene which encodes for the alpha domain of B-galactosidase that acts as a reporter protein in our | + | Improvement of LacZ alpha gene which encodes for the alpha domain of B-galactosidase that acts as a reporter protein in our diagnostic circuit : |
Lactose converting enzyme (B-gal) beta-galactosidase that is originated from bacteria And have been studied as a reporter protein for many years ,It's a polypeptide of 1029 amino acid, and its tetramerization carries out the functional form of the enzyme. | Lactose converting enzyme (B-gal) beta-galactosidase that is originated from bacteria And have been studied as a reporter protein for many years ,It's a polypeptide of 1029 amino acid, and its tetramerization carries out the functional form of the enzyme. | ||
| Line 22: | Line 23: | ||
The deletion of the tetramerization domain which is the N-terminal region spanning the first 50 residues will abolish its enzymatic activity, which could be fully restored by the alpha complementation of the intact N- terminal portion of the (Beta-gal) this alpha complementation is detected by using a beta-galactosidase colorless substrate as X gal to be cleaved into a dark blue product. | The deletion of the tetramerization domain which is the N-terminal region spanning the first 50 residues will abolish its enzymatic activity, which could be fully restored by the alpha complementation of the intact N- terminal portion of the (Beta-gal) this alpha complementation is detected by using a beta-galactosidase colorless substrate as X gal to be cleaved into a dark blue product. | ||
| − | This year we employed this approach to indicate the presence of phenylalanine within our whole cell biosensor on the test line with media containing X gal substrate of our platform to diagnose phenylketonuria | + | This year we employed this approach to indicate the presence of phenylalanine within our whole cell biosensor on the test line with media containing X gal substrate of our platform to diagnose phenylketonuria. |
| − | [[ | + | [[File:wcb.png| ]] |
| + | Figure 1. (shows the diagnostic whole cell-based biosensor after tagging lacZ alpha with kp-sp.) | ||
| + | |||
| + | |||
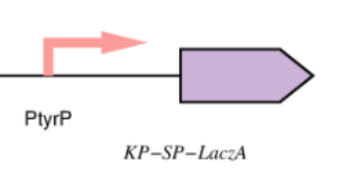
| + | [[Image:Lacz_Improv.png|thumb|right|Figure(2) Shows an SBOL demonstrating Lacz Improvement using Kp-Sp tagging ]] | ||
| + | |||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | |||
| + | |||
| + | ==Experimental validation of the improvement== | ||
| + | [[File:capture7.png|right|]] | ||
| + | <br><br><br><br><br><br><br> | ||
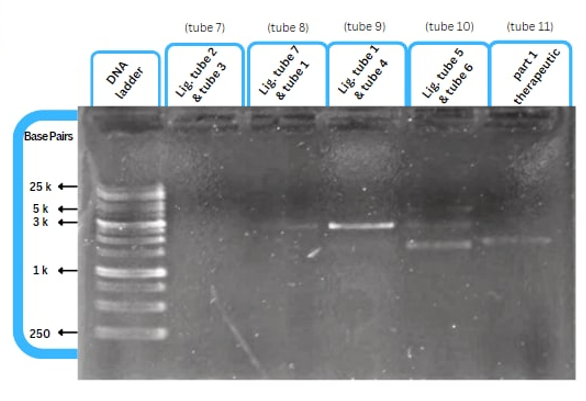
| + | This figure shows an experimental characterization of this part as it's validated through gel electrophoresis as it is in lane 3. The running part (ordered from IDT) included KP-SP and lacZ alpha. | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | [[File:cs1.png|ag1.png]] | ||
| + | <br><br> | ||
| + | This figure on the left (Phe-ve/X-gal +ve) shows that the WCB emits a low signal of the blue color at 0 mg/dL of phenylalanine. The results are shown to the right (Phe-ve/X-gal +ve), which shows that the WCB emits a negligible signal of the blue color at 0 mg/dL of phenylalanine. which is also a background noise from the circuit but with a very low signal after addition of the regulatory circuit. This iteration was essential as it proved the concept of background noise without using the regulatory circuit unless the result after transforming it. | ||
| + | [[File:without.png|without.png]] | ||
| + | <br><br><br> | ||
| + | For the previous part without improvement, the plate showed an incomplete negative percentage of dominant control color in the test sample. To confirm the results, we plotted the absorbance (nM) of both the tested sample and the control sample (standard positive) as they showed slight overlapping ranges of wavelength (nM). | ||
| + | |||
| + | [[File:with.png|right|]] | ||
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | For the improved part after transforming the regulatory circuit (kink turn- cas12g), the plate showed a negative percentage of dominant control color in the test sample. To confirm the results, we plotted the absorbance (nM) of both the improved sample and the control sample as they showed different ranges of wavelength (nM). | ||
=== Improvement by Directed Evolution === | === Improvement by Directed Evolution === | ||
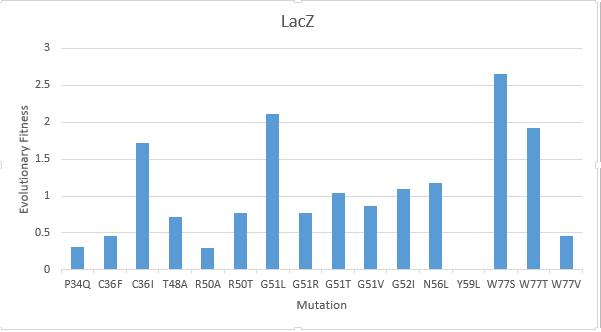
| − | After creating a multiple sequence alignment of the protein sequence and predicting mutational landscapes, the effect of these mutations on the evolutionary fitness of the protein is tested. The prediction of the mutational landscape by saturation mutagenesis of the LacZ protein. The (W77S ) mutation, as depicted in the chart, had the greatest score when compared to other mutations. On the other hand, it's clear that the (Y59L) had the least evolutionary fitness for LacZ protein. As displayed in Figure( | + | After creating a multiple sequence alignment of the protein sequence and predicting mutational landscapes, the effect of these mutations on the evolutionary fitness of the protein is tested. The prediction of the mutational landscape by saturation mutagenesis of the LacZ protein. The (W77S ) mutation, as depicted in the chart, had the greatest score when compared to other mutations. On the other hand, it's clear that the (Y59L) had the least evolutionary fitness for LacZ protein. As displayed in Figure(3) |
| + | [[Image:Lacz.png|thumb|Right|Figure(3) shows the mutational landscape of the LacZ protein ]] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | ==Literature Characterization== | ||
| + | According to the results, The ideal pH and temperature for Nf-LacZ are 40 °C and pH 6.5, respectively. The activity of the beta -galactosidase may also be impacted by metal ions. The metal ions K+, Mg2+, Ca2+, Zn2+, and Mn2+ were discovered to all be able to increase the enzymatic activity of Nf-LacZ.,and its Km was 05mmol/litre for Nf-LacZ, on the other hand the optimal temperature and PH for WspA are, respectively, 45 °C and pH 8.0, and Km value is close to to that of for WspA and this is showin in Nf-LacZ and WspA protein expression in vitro and analyzing its activity as shown in figures 4 and 5. | ||
| + | [[Image:LacZ-1.png|thumb|left|Figure.4 The recombinant Nf-LacZ was expressed by employing the E. coli expression system and its characters was analysed.]] | ||
| + | [[Image:LacZ-2.png|thumb|right|Figure.5 Expression of WspA and analysis of the protein polymerization state.]] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| − | [[ | + | ==Characterization by mathematical modeling== |
| − | <br><br><br> | + | We are using mathematical modeling to detect the increased level of phenylalanine (phe) in phenylketonuria patients in our diagnostic platform. It depends on a whole cell-based biosensor through a cascade of reactions to finally end by formation of β-galactosidase that turns the color into blue once bound to its substrate (X-gal) as mentioned in figure (4) and graph (1). |
| + | |||
| + | [[File:modell1.png|Right|]] | ||
| + | <br> | ||
| + | Figure (4) represents the cascade of reactions in whole cell-based biosensor model. | ||
| + | [[File:modell11.png|Right|]] | ||
| + | <br><br><br> | ||
| + | Graph(1) illustrates a direct relation between biomarker and beta-galactosidase ,so as the biomarker increases, the released amount of beta-galactosidase increases till it reaches constant value after about 30 time units. Therefore, the maximum amount of the biomarker releases the maximum amount of beta-galactosidase. | ||
==References== | ==References== | ||
| + | 1.Liu, W., Cui, L., Xu, H., Zhu, Z. & Gao, X. Flexibility-rigidity coordination of the dense exopolysaccharide matrix in terrestrial cyanobacteria acclimated to periodic desiccation. Appl. Environ. Microbiol. 83, e01619–17 (2017). | ||
| + | 2.Wright, D. J. et al. UV irradiation and desiccation modulate the three-dimensional extracellular matrix of Nostoc commune (Cyanobacteria). J. Biol. Chem. 280, 40271–40281 (2005). | ||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Latest revision as of 23:30, 11 October 2022
lacZ alpha
Part Description
The LacZ-alpha fragment is encoded by this region and is derived from the pUC19 cloning vector. Because it is smaller than the LacZ-omega region, the LacZ-alpha fragment can be easily incorporated into a plasmid when the two non-functional LacZ gene fragments (alpha and omega) are co-expressed. X-gal (5-bromo-4-chloro-3-indoyl-d-galactopyranoside), a soluble colourless molecule that is a substrate of ß-galactosidase and generates a blue product when cleaved, is used to detect the alpha-complementation
Usage
alpha domain of ß-galactosidase (our reporter protein) which is produced in response to high levels of phenylalanine and generates a blue product when X-gal is cleaved which is a soluble colorless molecule that is a substrate of ß-galactosidase and generates a blue product when cleaved, is used to detect the alpha-complementation. We used beta-galactosidase enzyme to turn the color into blue once increased level of phenylalanine is detected via exported extracellularly via tagging by kp-sp to bin with its substrate (X-gal).
Improvements
We improved the part BBa_E0033 by :-
Improvement by KP-SP tagging
This year, we enhanced the LacZ alpha gene by incorporating a peptide signal that controls the secretion of B galactosidase extracellularly (KP-SP tagging) in an effort to improve the cleavage capacity of the X gal, heighten the intensity of the dark blue color emitted by the X gal product, and reduce the amount of time required to receive the results after performing the test on the chip. as shown in figure 1 and figure 2.
Improvement of LacZ alpha gene which encodes for the alpha domain of B-galactosidase that acts as a reporter protein in our diagnostic circuit :
Lactose converting enzyme (B-gal) beta-galactosidase that is originated from bacteria And have been studied as a reporter protein for many years ,It's a polypeptide of 1029 amino acid, and its tetramerization carries out the functional form of the enzyme.
The deletion of the tetramerization domain which is the N-terminal region spanning the first 50 residues will abolish its enzymatic activity, which could be fully restored by the alpha complementation of the intact N- terminal portion of the (Beta-gal) this alpha complementation is detected by using a beta-galactosidase colorless substrate as X gal to be cleaved into a dark blue product.
This year we employed this approach to indicate the presence of phenylalanine within our whole cell biosensor on the test line with media containing X gal substrate of our platform to diagnose phenylketonuria.
Figure 1. (shows the diagnostic whole cell-based biosensor after tagging lacZ alpha with kp-sp.)
Experimental validation of the improvement
This figure shows an experimental characterization of this part as it's validated through gel electrophoresis as it is in lane 3. The running part (ordered from IDT) included KP-SP and lacZ alpha.

This figure on the left (Phe-ve/X-gal +ve) shows that the WCB emits a low signal of the blue color at 0 mg/dL of phenylalanine. The results are shown to the right (Phe-ve/X-gal +ve), which shows that the WCB emits a negligible signal of the blue color at 0 mg/dL of phenylalanine. which is also a background noise from the circuit but with a very low signal after addition of the regulatory circuit. This iteration was essential as it proved the concept of background noise without using the regulatory circuit unless the result after transforming it.

For the previous part without improvement, the plate showed an incomplete negative percentage of dominant control color in the test sample. To confirm the results, we plotted the absorbance (nM) of both the tested sample and the control sample (standard positive) as they showed slight overlapping ranges of wavelength (nM).
For the improved part after transforming the regulatory circuit (kink turn- cas12g), the plate showed a negative percentage of dominant control color in the test sample. To confirm the results, we plotted the absorbance (nM) of both the improved sample and the control sample as they showed different ranges of wavelength (nM).
Improvement by Directed Evolution
After creating a multiple sequence alignment of the protein sequence and predicting mutational landscapes, the effect of these mutations on the evolutionary fitness of the protein is tested. The prediction of the mutational landscape by saturation mutagenesis of the LacZ protein. The (W77S ) mutation, as depicted in the chart, had the greatest score when compared to other mutations. On the other hand, it's clear that the (Y59L) had the least evolutionary fitness for LacZ protein. As displayed in Figure(3)
Literature Characterization
According to the results, The ideal pH and temperature for Nf-LacZ are 40 °C and pH 6.5, respectively. The activity of the beta -galactosidase may also be impacted by metal ions. The metal ions K+, Mg2+, Ca2+, Zn2+, and Mn2+ were discovered to all be able to increase the enzymatic activity of Nf-LacZ.,and its Km was 05mmol/litre for Nf-LacZ, on the other hand the optimal temperature and PH for WspA are, respectively, 45 °C and pH 8.0, and Km value is close to to that of for WspA and this is showin in Nf-LacZ and WspA protein expression in vitro and analyzing its activity as shown in figures 4 and 5.
Characterization by mathematical modeling
We are using mathematical modeling to detect the increased level of phenylalanine (phe) in phenylketonuria patients in our diagnostic platform. It depends on a whole cell-based biosensor through a cascade of reactions to finally end by formation of β-galactosidase that turns the color into blue once bound to its substrate (X-gal) as mentioned in figure (4) and graph (1).
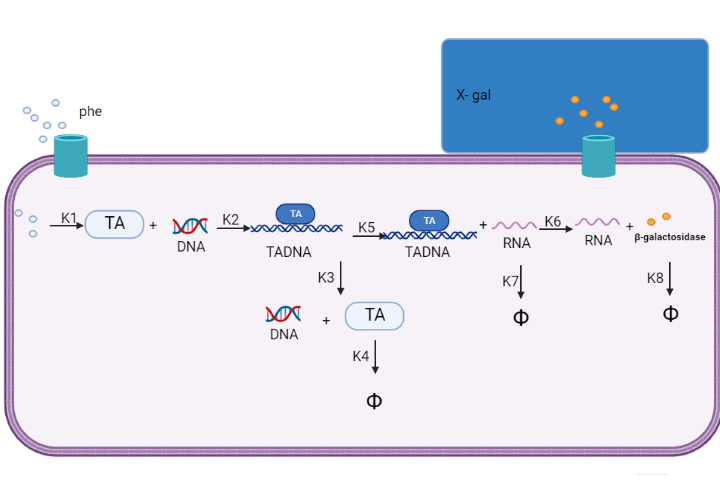
Figure (4) represents the cascade of reactions in whole cell-based biosensor model.
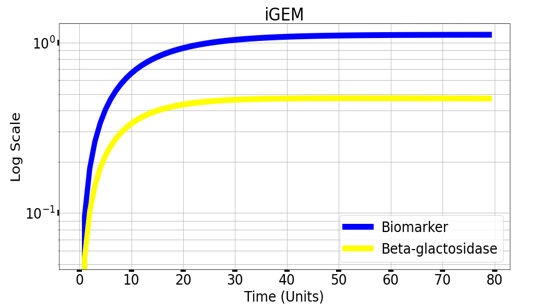
Graph(1) illustrates a direct relation between biomarker and beta-galactosidase ,so as the biomarker increases, the released amount of beta-galactosidase increases till it reaches constant value after about 30 time units. Therefore, the maximum amount of the biomarker releases the maximum amount of beta-galactosidase.
References
1.Liu, W., Cui, L., Xu, H., Zhu, Z. & Gao, X. Flexibility-rigidity coordination of the dense exopolysaccharide matrix in terrestrial cyanobacteria acclimated to periodic desiccation. Appl. Environ. Microbiol. 83, e01619–17 (2017). 2.Wright, D. J. et al. UV irradiation and desiccation modulate the three-dimensional extracellular matrix of Nostoc commune (Cyanobacteria). J. Biol. Chem. 280, 40271–40281 (2005).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 93
Illegal XhoI site found at 144 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]