Difference between revisions of "Part:BBa K3781017"
| (12 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K3781017 short</partinfo> | <partinfo>BBa_K3781017 short</partinfo> | ||
| + | |||
| + | [[File:T--TU Kaiserslautern--Projektlogo.png|100px|right|MocloMania]] | ||
<p>This part codes for a <b>Strep-tag II</b> that is fused to a downstream <b>8His-tag</b>.<br>The Strep-tag II is part of a <b>protein purification system</b> that in its principle it relies on the high affinity binding of the homo-tetrameric protein <b>streptavidin</b>, first isolated from the bacterium <i>Streptomyces avidinii</i>, to the vitamin <b>biotin</b>.<ref><b>W A Hendrickson</b>: Crystal Structure of Core Streptavidin Determined from Multiwavelength Anomalous Diffraction of Synchrotron Radiation. In: Proceedings of the National Academy of Sciences. 86 (7), 1989, S. 2190–4.</ref> The binding of these two biomolecules is one of the strongest non-covalent interactions observed in nature and inspired the creation of many derivatives with even further enhanced binding capabilities.<ref><b>Green NM</b> (1975). "Avidin". Advances in Protein Chemistry. 29: 85–133. doi:10.1016/s0065-3233(08)60411-8. ISBN 9780120342297. PMID 237414.</ref> German biotech company <b>IBA Life Sciences</b> developed a <b>synthetic</b> eight amino acid peptide called <b>Strep-tag II</b> which binds to the specifically engineered streptavidin derivative <b>Strep-Tactin</b> with high specificity.<ref>https://www.iba-lifesciences.com/strep-tag</ref> Thus, fusing a gene of interest to a Strep-tag II allows for efficient purification of the target protein by <b>affinity chromatography</b> over immobilized Strep-Tactin. The bound target protein can later be eluted with <b>desthiobiotin</b>.</p> | <p>This part codes for a <b>Strep-tag II</b> that is fused to a downstream <b>8His-tag</b>.<br>The Strep-tag II is part of a <b>protein purification system</b> that in its principle it relies on the high affinity binding of the homo-tetrameric protein <b>streptavidin</b>, first isolated from the bacterium <i>Streptomyces avidinii</i>, to the vitamin <b>biotin</b>.<ref><b>W A Hendrickson</b>: Crystal Structure of Core Streptavidin Determined from Multiwavelength Anomalous Diffraction of Synchrotron Radiation. In: Proceedings of the National Academy of Sciences. 86 (7), 1989, S. 2190–4.</ref> The binding of these two biomolecules is one of the strongest non-covalent interactions observed in nature and inspired the creation of many derivatives with even further enhanced binding capabilities.<ref><b>Green NM</b> (1975). "Avidin". Advances in Protein Chemistry. 29: 85–133. doi:10.1016/s0065-3233(08)60411-8. ISBN 9780120342297. PMID 237414.</ref> German biotech company <b>IBA Life Sciences</b> developed a <b>synthetic</b> eight amino acid peptide called <b>Strep-tag II</b> which binds to the specifically engineered streptavidin derivative <b>Strep-Tactin</b> with high specificity.<ref>https://www.iba-lifesciences.com/strep-tag</ref> Thus, fusing a gene of interest to a Strep-tag II allows for efficient purification of the target protein by <b>affinity chromatography</b> over immobilized Strep-Tactin. The bound target protein can later be eluted with <b>desthiobiotin</b>.</p> | ||
| − | <p>The Strep-tag II encoded in this part is fused to an <b>8His-tag</b>, a <b>polyhistidine-tag</b> consisting of <b>eight</b> consecutive <b>histidine residues</b>. The histidine sidechain contains a heterocyclic <b>imidazole</b> ring that is negatively charged in neutral to basic conditions and can <b>coordinate with metal ions</b> with very high affinity.<ref><b>Creighton, T.E.</b> Proteins: Structures and Molecular Properties, 2nd ed.; W. H. Freeman: New York, NY, USA, 1992.</ref> Thus, equipping a target protein with a terminal histidine rich peptide sequence allows for purification of the protein via <b>immobilized metal ion affinity chromatography</b>.<ref><b>Bornhorst JA</b>, Falke JJ. Purification of proteins using polyhistidine affinity tags. Methods Enzymol. 2000;326:245-254. doi:10.1016/s0076-6879(00)26058-8</ref> | + | <p>The Strep-tag II encoded in this part is fused to an <b>8His-tag</b>, a <b>polyhistidine-tag</b> consisting of <b>eight</b> consecutive <b>histidine residues</b>. The histidine sidechain contains a heterocyclic <b>imidazole</b> ring that is negatively charged in neutral to basic conditions and can <b>coordinate with metal ions</b> with very high affinity.<ref><b>Creighton, T.E.</b> Proteins: Structures and Molecular Properties, 2nd ed.; W. H. Freeman: New York, NY, USA, 1992.</ref> Thus, equipping a target protein with a terminal histidine rich peptide sequence allows for purification of the protein via <b>immobilized metal ion affinity chromatography</b>.<ref><b>Bornhorst JA</b>, Falke JJ. Purification of proteins using polyhistidine affinity tags. Methods Enzymol. 2000;326:245-254. doi:10.1016/s0076-6879(00)26058-8</ref> The bound target protein can later be eluted with <b>excess imidazole</b>.</p> |
| + | <p>By incorporating two different protein tags in one, this basic part generates even more <b>flexibility</b> when it comes to downstream purification procedures. As a <b>B5 part</b>, this part is furthermore meant to occupy the most downstream position in the MocloMania cloning frame and is thus equipped with an <b>additional stop codon</b>.</p> | ||
| Line 16: | Line 19: | ||
<html> | <html> | ||
| − | <b>plasmid backbone</b> | + | <b>plasmid backbone</b> <a href="https://parts.igem.org/Part:BBa_K3781104">pAGM1301</a> |
</html> | </html> | ||
<br> | <br> | ||
<br> | <br> | ||
<h1>Data</h1> | <h1>Data</h1> | ||
| + | |||
| + | We were able to <b>successfully clone</b> this basic part into its respective L0 plasmid backbone and to <b>confirm</b> the integrity of the L0 construct via <b>restriction digest</b> and gel electrophoresis, see <i>Figure 1</i>. Furthermore, we were able to include it into a <b>L1 construct</b>, proving its correct adaptation towards MoClo assembly, see <i>Figure 2</i>. | ||
| + | |||
| + | |||
| + | <div><ul> | ||
| + | <li style="display: inline-block;"> [[File:T--TU Kaiserslautern--Geldbild L0-Strep8His-B5.png|thumb|none|400px|<b>Figure 1</b> | <b>Test digest of L0_Strep8His_B5</b> using <i>SapI</i> and <i>BstEII</i><br> | ||
| + | <b>1</b> | <b>L0_Strep8His_B5</b> | 1565 + 581 + 165 bp<br> | ||
| + | <b>2</b> | <html><a href="https://parts.igem.org/Part:BBa_K3781104">pAGM1301</a></html> | 1565 + 699 + 581 bp<br> | ||
| + | <b>L</b> | Thermofischer GeneRuler Plus Ladder [bp]]] </li> | ||
| + | <li style="display: inline-block;"> [[File:T--TU Kaiserslautern--Gelbild 3xHAslashsAP RBD Strep8His.png|thumb|none|400px|<b>Figure 2</b> | <b>Test digest of L1 constructs</b> using <i>PstI</i><br> | ||
| + | <b>1</b> | <html><a href="https://parts.igem.org/Part:BBa_K3781213">L1_sAP_RBD_<b>Strep8His</b></a></html> | 3894 + 1323 + 991 + 991 + 757 bp<br> | ||
| + | <b>2</b> | <html><a href="https://parts.igem.org/Part:BBa_K3781202">L1_3xHA_RBD_<b>Strep8His</b></a></html> | 3867 + 1323 + 991 + 991 + 757 bp<br> | ||
| + | <b>L</b> | Thermofischer GeneRuler Plus Ladder [bp]]] </li> | ||
| + | </ul></div> | ||
| + | |||
| + | |||
| + | A variety of different L1 constructs assembled with L0-Strep8His_B5 have been <b>successfully transfected</b> into <i>Leishmania</i>, resulting in recombinant protein expression that could be observed after <b>immunostaining</b> on <b>western blot</b> for both staining against <b>RBD</b>, see <i>Figure 3</i>, as well as against the <b>polyhistidine-tag</b>, see <i>Figure 4</i>. This verifies the <b>cohesive nature</b> of our expressed fusion protein and proves the <b>functionality</b> of the Strep8His-tag regarding <b>His-tag antibody detection</b>. | ||
| + | |||
| + | |||
| + | <div><ul> | ||
| + | <li style="display: inline-block;"> [[File:T--TU Kaiserslautern--Blot L1 sAP RBD mCerulean RBD 02.10.png|thumb|none|600px|<b>Figure 3</b> | <b>Immunoblot</b> of L1 transfected Leishmania | stained against <b>RBD</b><br> | ||
| + | <b>1</b> | <html><a href="https://parts.igem.org/Part:BBa_K3781207">L1_sAP_RBD_mCerulean</a></html> | 51.9 kDa<br> | ||
| + | <b>2</b> | <html><a href="https://parts.igem.org/Part:BBa_K378123">L1_sAP_RBD_<b>Strep8His</b></a></html> | 27.7 kDa<br> | ||
| + | <b>n.c.</b> | negative control | untransfected <i>Leishmania</i> culture<br> | ||
| + | <b>p.c.</b> | RBD-GFP | 54 kDa<br> | ||
| + | <b>L</b> | Thermofischer PageRuler Protein Ladder [kDa]<br> | ||
| + | 1. AB | ms <b>anti-RBD</b> | 1:2,000<br> | ||
| + | 2. AB | rb <b>anti-ms HRP</b> | 1:10,000<br> ]] </li> | ||
| + | <li style="display: inline-block;"> [[File:T--TU Kaiserslautern--Blot L1 sAP RBD Strep8His L1 sAP RBD HA8His His 01-10.png|thumb|none|600px|<b>Figure 4</b> | <b>Immunoblot</b> of L1 transfected Leishmania | stained against <b>His</b> | ||
| + | <p><b>1</b> | <html><a href="https://parts.igem.org/Part:BBa_K378206">L1_sAP_RBD_HA8His</a></html> | 28 kDa<br> | ||
| + | <b>2</b> | <html><a href="https://parts.igem.org/Part:BBa_K378213">L1_sAP_RBD_<b>Strep8His</b></a></html> | 27.7 kDa<br> | ||
| + | <b>p.c.</b> | exemplary His-tagged protein | 23 kDa<br> | ||
| + | <b>n.c.</b> | culture transfected with empty L1 expression vector<br> | ||
| + | <b>L</b> | Thermo Scientific PageRuler Protein Ladder [kDa]<br> | ||
| + | 1. AB | ms <b>anti-His</b> | 1:4,000<br> | ||
| + | 2. AB | rb <b>anti-ms HRP</b> | 1:10,000</p>]] </li> | ||
| + | </ul></div> | ||
| + | |||
| + | |||
| + | To this day, detection against <b>Strep-antibodies</b> has remained unsuccessful. Unlike most other antibodies, the Strep-antibodies <b>available</b> in our <b>working group</b> were coupled to <b>alkaline phosphatase</b>, requiring a different detection mechanism than <b>horseraddish peroxidase-mediated chemiluminescence</b>. We suppose that expression signals were <b>too weak</b> for visual alkaline phosphatase detection using <b>BCIP</b> and <b>NBT</b>. In the future, acquisition of <b>HRP-tagged anti-Strep-tag II antibodies</b> might facilitate Strep-based detection. | ||
| Line 30: | Line 73: | ||
<p>We furthermore provide a specifically domesticated <b>Leishmania expression vector</b>, named <a href="https://parts.igem.org/Part:BBa_K3781105"><b>weird_plex</b></a>, which will package your fusion construct into a functional <b>transcriptional unit</b> that is optimized for high expression in Leishmania. | <p>We furthermore provide a specifically domesticated <b>Leishmania expression vector</b>, named <a href="https://parts.igem.org/Part:BBa_K3781105"><b>weird_plex</b></a>, which will package your fusion construct into a functional <b>transcriptional unit</b> that is optimized for high expression in Leishmania. | ||
<p>The best part? Because of the type IIS restriction properties and the specifity of the generated overhangs, restriction and ligation of your construct can all happen <b>simultaneously</b> in a simple <b>one-step</b>, <b>one-pot reaction</b>. This will safe you a lot of time and frustration in your cloning endeavours!</p> | <p>The best part? Because of the type IIS restriction properties and the specifity of the generated overhangs, restriction and ligation of your construct can all happen <b>simultaneously</b> in a simple <b>one-step</b>, <b>one-pot reaction</b>. This will safe you a lot of time and frustration in your cloning endeavours!</p> | ||
| − | <p>Do we have your attention? | + | <p>Do we have your attention? In the <b>table below</b> you can find some basic information on how our cloning system, along with most other MoClo systems, is set up. Please feel free to check out our wiki to find more information on <a href="https://2021.igem.org/Team:TU_Kaiserslautern/Description">Leishmania and Modular Cloning</a> as well as to understand how this basic part integrates into our <a href="https://2021.igem.org/Team:TU_Kaiserslautern/Part_Collection"><b>part collection</b></a>. See you there!</p> |
</html> | </html> | ||
| − | <span class='h3bb'>Sequence and Features</span> | + | {| class="wikitable" |
| + | |+ style="text-align: left;" | <b>MOCLO</b> | Important nomenclature and parameters | ||
| + | |- | ||
| + | | <b>Level</b> || <b>What does this level contain?</b> || <b>antibiotic resistance</b> || <b>Enzyme used for ligation</b> | ||
| + | |- | ||
| + | | <b>L0</b> || The foundation to every MoClo construct which are basic <b>genetic units</b>, such as coding sequences, promoters, terminators || spectinomycin || BbsI | ||
| + | |- | ||
| + | | <b>L1</b> || Several L0 parts assembled into a functional <b>transcriptional unit</b>, e.g. consisting of promoter, coding region and terminator || ampicillin || BsaI | ||
| + | |- | ||
| + | | <b>L2</b> || Multiple transcriptional units added into one <b>multi-gene construct</b>, e.g. a protein of interest fused to a selection marker || kanamycin || BbsI | ||
| + | |} | ||
| + | |||
| + | |||
| + | <span class='h3bb'><h1>Sequence and Features</h1></span> | ||
<partinfo>BBa_K3781017 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3781017 SequenceAndFeatures</partinfo> | ||
Latest revision as of 03:22, 22 October 2021
Strep8His, MocloMania B5
This part codes for a Strep-tag II that is fused to a downstream 8His-tag.
The Strep-tag II is part of a protein purification system that in its principle it relies on the high affinity binding of the homo-tetrameric protein streptavidin, first isolated from the bacterium Streptomyces avidinii, to the vitamin biotin.[1] The binding of these two biomolecules is one of the strongest non-covalent interactions observed in nature and inspired the creation of many derivatives with even further enhanced binding capabilities.[2] German biotech company IBA Life Sciences developed a synthetic eight amino acid peptide called Strep-tag II which binds to the specifically engineered streptavidin derivative Strep-Tactin with high specificity.[3] Thus, fusing a gene of interest to a Strep-tag II allows for efficient purification of the target protein by affinity chromatography over immobilized Strep-Tactin. The bound target protein can later be eluted with desthiobiotin.
The Strep-tag II encoded in this part is fused to an 8His-tag, a polyhistidine-tag consisting of eight consecutive histidine residues. The histidine sidechain contains a heterocyclic imidazole ring that is negatively charged in neutral to basic conditions and can coordinate with metal ions with very high affinity.[4] Thus, equipping a target protein with a terminal histidine rich peptide sequence allows for purification of the protein via immobilized metal ion affinity chromatography.[5] The bound target protein can later be eluted with excess imidazole.
By incorporating two different protein tags in one, this basic part generates even more flexibility when it comes to downstream purification procedures. As a B5 part, this part is furthermore meant to occupy the most downstream position in the MocloMania cloning frame and is thus equipped with an additional stop codon.
size 2.3 kDa
function affinity purification tag
amino acid sequence WSHPQFEK GG HHHHHHHH
cloning position B5
plasmid backbone pAGM1301
Data
We were able to successfully clone this basic part into its respective L0 plasmid backbone and to confirm the integrity of the L0 construct via restriction digest and gel electrophoresis, see Figure 1. Furthermore, we were able to include it into a L1 construct, proving its correct adaptation towards MoClo assembly, see Figure 2.
-
 Figure 1 | Test digest of L0_Strep8His_B5 using SapI and BstEII
Figure 1 | Test digest of L0_Strep8His_B5 using SapI and BstEII
1 | L0_Strep8His_B5 | 1565 + 581 + 165 bp
2 | pAGM1301 | 1565 + 699 + 581 bp
L | Thermofischer GeneRuler Plus Ladder [bp] -
 Figure 2 | Test digest of L1 constructs using PstI
Figure 2 | Test digest of L1 constructs using PstI
1 | L1_sAP_RBD_Strep8His | 3894 + 1323 + 991 + 991 + 757 bp
2 | L1_3xHA_RBD_Strep8His | 3867 + 1323 + 991 + 991 + 757 bp
L | Thermofischer GeneRuler Plus Ladder [bp]
A variety of different L1 constructs assembled with L0-Strep8His_B5 have been successfully transfected into Leishmania, resulting in recombinant protein expression that could be observed after immunostaining on western blot for both staining against RBD, see Figure 3, as well as against the polyhistidine-tag, see Figure 4. This verifies the cohesive nature of our expressed fusion protein and proves the functionality of the Strep8His-tag regarding His-tag antibody detection.
-
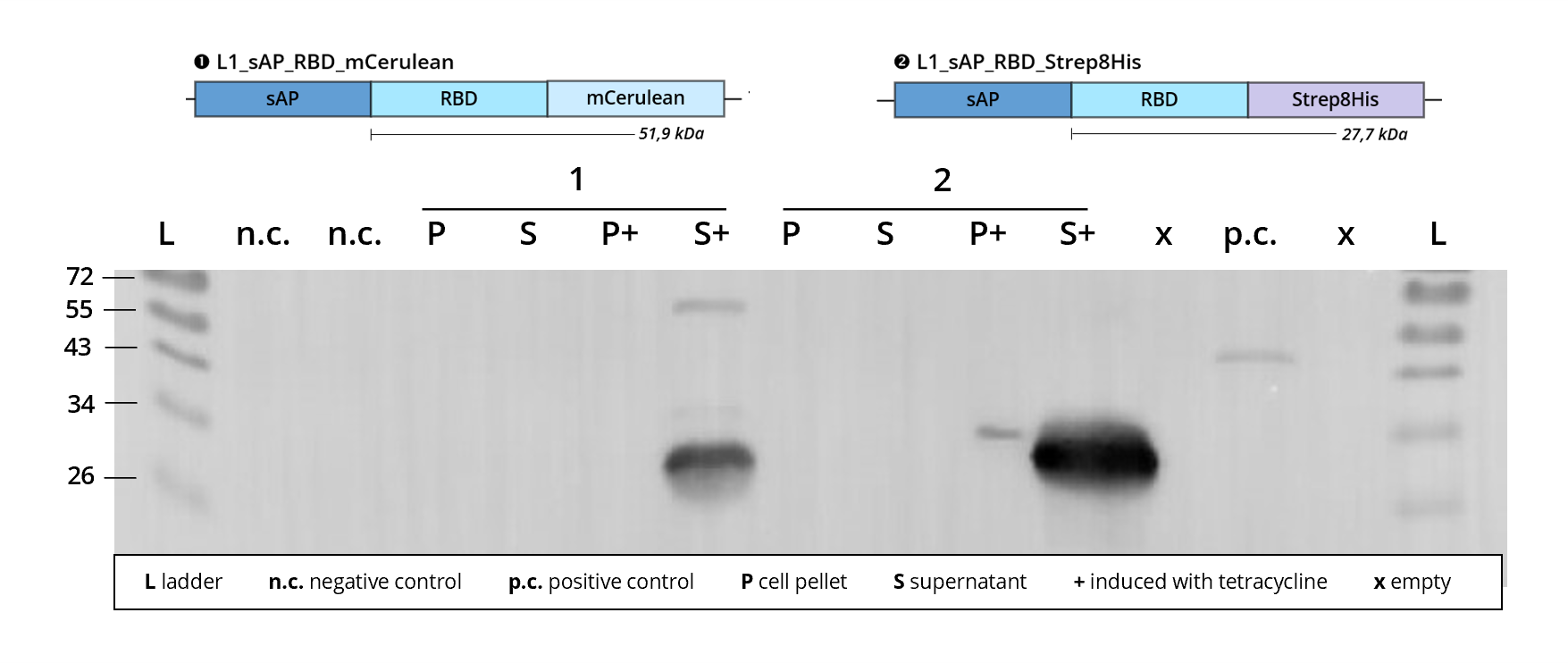 Figure 3 | Immunoblot of L1 transfected Leishmania | stained against RBD
Figure 3 | Immunoblot of L1 transfected Leishmania | stained against RBD
1 | L1_sAP_RBD_mCerulean | 51.9 kDa
2 | L1_sAP_RBD_Strep8His | 27.7 kDa
n.c. | negative control | untransfected Leishmania culture
p.c. | RBD-GFP | 54 kDa
L | Thermofischer PageRuler Protein Ladder [kDa]
1. AB | ms anti-RBD | 1:2,000
2. AB | rb anti-ms HRP | 1:10,000 -
 Figure 4 | Immunoblot of L1 transfected Leishmania | stained against His
Figure 4 | Immunoblot of L1 transfected Leishmania | stained against His1 | L1_sAP_RBD_HA8His | 28 kDa
2 | L1_sAP_RBD_Strep8His | 27.7 kDa
p.c. | exemplary His-tagged protein | 23 kDa
n.c. | culture transfected with empty L1 expression vector
L | Thermo Scientific PageRuler Protein Ladder [kDa]
1. AB | ms anti-His | 1:4,000
2. AB | rb anti-ms HRP | 1:10,000
To this day, detection against Strep-antibodies has remained unsuccessful. Unlike most other antibodies, the Strep-antibodies available in our working group were coupled to alkaline phosphatase, requiring a different detection mechanism than horseraddish peroxidase-mediated chemiluminescence. We suppose that expression signals were too weak for visual alkaline phosphatase detection using BCIP and NBT. In the future, acquisition of HRP-tagged anti-Strep-tag II antibodies might facilitate Strep-based detection.
The MocloMania collection
This basic part is part of the MocloMania collection, the very first collection of genetic parts specifically designed and optimized for Modular Cloning assembly and recombinant protein expression in the protozoan parasite Leishmania tarentolae.
Are you trying to express complexly glycosylated proteins? Large antibody side chains? Human proteins that require accurate post-translational modification? Then Leishmania might be just the right organism for you! Leishmania tarentolae’s glycosylation patterns resemble those of human cells more closely than any other microbial expression host, while still delivering all the benefits of microbial production systems like easy transfection and cultivation.[6] So instead of relying on mammalian cell lines, try considering Leishmania as your new expression host of choice!
Our MocloMania collection will allow you to easily modify your protein of choice and make it suitable for downstream detection and purification procedures - all thanks to the help of Modular Cloning. This cloning system was first established by Weber et al. in 2011 and relies on the ability of type IIS restriction enzymes to cut DNA outside of their recognition sequence, hereby generating four nucleotide overhangs.[7] Every basic part in our collection is equipped with a specified set of overhangs that assign it to its designated position within the reading frame. These so-called cloning positions are labelled B2-B5 from upstream to downstream. By filling all positions with the basic parts of your choice, you can easily generate variable genetic constructs that code for the fusion protein of your desire.
We furthermore provide a specifically domesticated Leishmania expression vector, named weird_plex, which will package your fusion construct into a functional transcriptional unit that is optimized for high expression in Leishmania.
The best part? Because of the type IIS restriction properties and the specifity of the generated overhangs, restriction and ligation of your construct can all happen simultaneously in a simple one-step, one-pot reaction. This will safe you a lot of time and frustration in your cloning endeavours!
Do we have your attention? In the table below you can find some basic information on how our cloning system, along with most other MoClo systems, is set up. Please feel free to check out our wiki to find more information on Leishmania and Modular Cloning as well as to understand how this basic part integrates into our part collection. See you there!
| Level | What does this level contain? | antibiotic resistance | Enzyme used for ligation |
| L0 | The foundation to every MoClo construct which are basic genetic units, such as coding sequences, promoters, terminators | spectinomycin | BbsI |
| L1 | Several L0 parts assembled into a functional transcriptional unit, e.g. consisting of promoter, coding region and terminator | ampicillin | BsaI |
| L2 | Multiple transcriptional units added into one multi-gene construct, e.g. a protein of interest fused to a selection marker | kanamycin | BbsI |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Reference Literature
- ↑ W A Hendrickson: Crystal Structure of Core Streptavidin Determined from Multiwavelength Anomalous Diffraction of Synchrotron Radiation. In: Proceedings of the National Academy of Sciences. 86 (7), 1989, S. 2190–4.
- ↑ Green NM (1975). "Avidin". Advances in Protein Chemistry. 29: 85–133. doi:10.1016/s0065-3233(08)60411-8. ISBN 9780120342297. PMID 237414.
- ↑ https://www.iba-lifesciences.com/strep-tag
- ↑ Creighton, T.E. Proteins: Structures and Molecular Properties, 2nd ed.; W. H. Freeman: New York, NY, USA, 1992.
- ↑ Bornhorst JA, Falke JJ. Purification of proteins using polyhistidine affinity tags. Methods Enzymol. 2000;326:245-254. doi:10.1016/s0076-6879(00)26058-8
- ↑ Langer T, Corvey C, Kroll K, Boscheinen O, Wendrich T, Dittrich W. Expression and purification of the extracellular domains of human glycoprotein VI (GPVI) and the receptor for advanced glycation end products (RAGE) from Rattus norvegicus in Leishmania tarentolae. Prep Biochem Biotechnol. 2017 Nov 26;47(10):1008-1015. doi: 10.1080/10826068.2017.1365252. Epub 2017 Aug 31. PMID: 28857681.
- ↑ Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S (2011) A Modular Cloning System for Standardized Assembly of Multigene Constructs. PLoS ONE 6(2): e16765. https://doi.org/10.1371/journal.pone.0016765