Difference between revisions of "Part:BBa K3799057"
(→Charactarization) |
(→Characterization) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3799057 short</partinfo> | <partinfo>BBa_K3799057 short</partinfo> | ||
| − | This is an improvement of the existing part [https://parts.igem.org/Part:BBa_K1022100 BBa_K1022100]. | + | This is an improvement of the existing part [https://parts.igem.org/Part:BBa_K1022100 BBa_K1022100] , by flipping the direction of p2 promoter to the opposite direction of pBAD promoter. |
| Line 31: | Line 31: | ||
<!-- --> | <!-- --> | ||
| − | === | + | ===Characterization=== |
| + | '''Introduction''' | ||
| + | We observed that there is leaky gene expression in the Unidirectional circuit. We modified the existing part to reduce the leaky gene expression. By flipping the gene we expect that the leaky gene expression will be reduced. To check our hypotheses, we have compared the expression of GFP in the unidirectional and the bidirectional genetic circuit. | ||
| − | First, we PCR extended the three segments (pBAD-agrC, P2-GFP,agrA,) of the unidirectional circuit with specific primers. | + | '''Experiment''' |
| + | |||
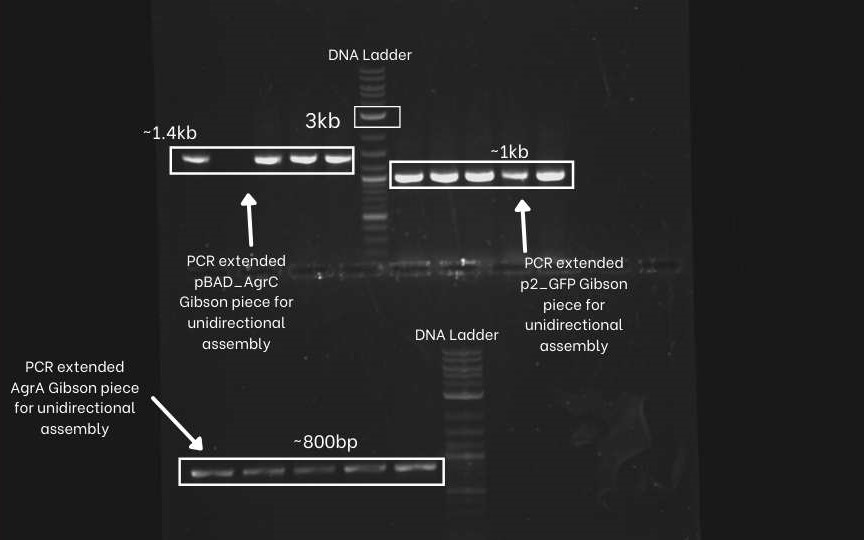
| + | First, we PCR extended the three DNA segments (pBAD-agrC, P2-GFP,agrA,) of the unidirectional circuit with specific primers. To confirm the success of PCR extension an Agarose Gel Electrophoresis was performed. As evident from the picture below, correct bands for all three amplified DNA segments were obtained. (Extended pBAD-agrC ~ 1.4 Kb, Extended P2-GFP ~ 1Kb, Extended agrA ~ 800bp and extended backbone~2kb. | ||
[[Image:T--IISER_Kolkata--uni_colony_pcr.jpg | thumb | center | 700 px |PCR extension of pBAD-agrC,P2-GFP,agrA for unidirectional assembly | [[Image:T--IISER_Kolkata--uni_colony_pcr.jpg | thumb | center | 700 px |PCR extension of pBAD-agrC,P2-GFP,agrA for unidirectional assembly | ||
]] | ]] | ||
[[Image:T--IISER_Kolkata--psb1c3.jpeg | thumb | center | 800 px |PCR extension of psb1C3 for unidirectional assembly]] | [[Image:T--IISER_Kolkata--psb1c3.jpeg | thumb | center | 800 px |PCR extension of psb1C3 for unidirectional assembly]] | ||
| − | + | After that, the same three segments (pBAD-agrC, P2-GFP, agrA, and psb1C3 plasmid backbone) were PCR amplified with different sets of specific primers. This extended overlap helps flip the P2-GFP fragments. From the gel electrophoresis image below. We can observe similar band sizes as above because the difference in length of the extension is very low. | |
[[Image:T--IISER_Kolkata--bidi_extension.jpg | thumb | center | 700 px |PCR extension of pBAD-agrC,P2-GFP,agrA for bidirectional assembly | [[Image:T--IISER_Kolkata--bidi_extension.jpg | thumb | center | 700 px |PCR extension of pBAD-agrC,P2-GFP,agrA for bidirectional assembly | ||
]] | ]] | ||
[[Image:T--IISER_Kolkata--AgrA_extension.jpg | thumb | center | 700 px | PCR extention of agrA segment | [[Image:T--IISER_Kolkata--AgrA_extension.jpg | thumb | center | 700 px | PCR extention of agrA segment | ||
]] | ]] | ||
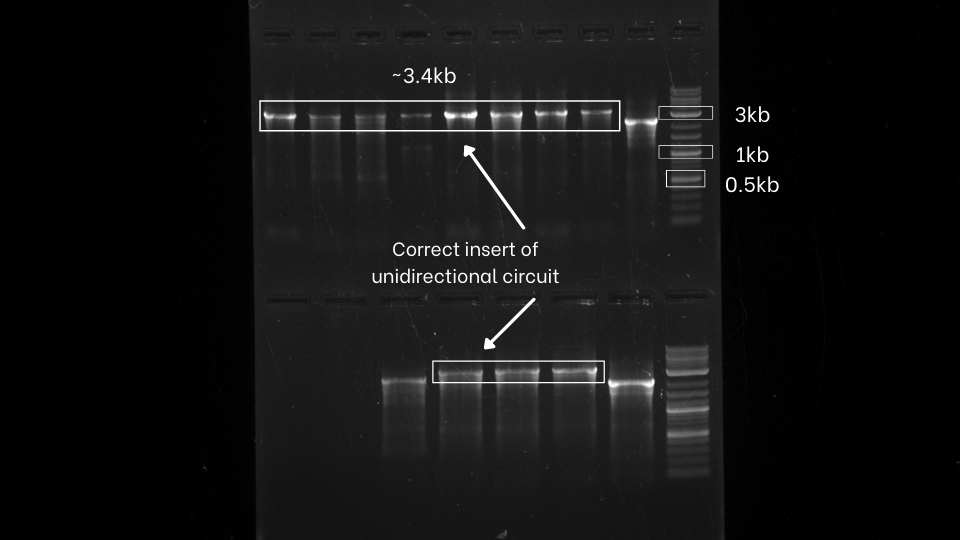
| − | After the successful extension of the segments, | + | After the successful extension of the segments, those extended fragments were assembled using Hi-Fi DNA assembly master mix. The reaction mixture was transformed in the E.Coil DH5a. After that, colony PCR of both the unidirectional and bidirectional circuit was performed using VF2 and VR primers to identify the bacterial colony with our desired gene circuits. |
| − | + | As evident from the colony PCR results, positive inserts for both unidirectional and bidirectional assemblies were obtained. | |
[[Image:T--IISER_Kolkata--uni_pcr_colony.jpg | thumb | center | 700 px | Colony PCR result of unidirectional assembly with VF2 and VR | [[Image:T--IISER_Kolkata--uni_pcr_colony.jpg | thumb | center | 700 px | Colony PCR result of unidirectional assembly with VF2 and VR | ||
]] | ]] | ||
| Line 52: | Line 56: | ||
| − | After the successful assembling of both the fragments, we planned to see the expression level of GFP in both bidirectional and unidirectional circuits. For that, | + | After the successful assembling of both the fragments, we planned to see the expression level of GFP in both bidirectional and unidirectional circuits. For that, first, the AIP-1 generator (BBa_K3799006) was expressed in 40ml of bacterial culture to obtain AIP-1 molecules. After expressing for 8-10 hours, the culture was centrifuged and the supernatant was filter-sterilized. The supernatant was lyophilized to concentrate the AIP-1 molecule. After full lyophilization, it was resuspended in 3 ml of NFW to make a stock solution of AIP1. |
| − | + | The stock solution of AIP-1 molecules was diluted to make solutions of different concentrations, from 0% to 100%, where 0% was the negative control and 100% was the one-third dilution of our stock solution. | |
| − | + | The two types of cells were incubated (one with unidirectional plasmid and the other with bidirectional plasmid) at 30℃ for 8 hours without AIP-1. | |
| − | + | The fluorescence intensity of those two types of cells in absence of AIP-1 molecules was measured at different time points and it was observed that there is a higher fluorescence intensity in cells with unidirectional circuits as compared to the cells with bidirectional circuits. This infers that the bidirectional circuit has less leaky gene expression than that of the unidirectional circuit. | |
[[Image:IISER_Kolkata--Bi-Intensity_vs_time.jpeg | thumb | center | 800 px | Fluorescence assay of bidirectional and unidirectional circuits without AIP-1 vs Time]] | [[Image:IISER_Kolkata--Bi-Intensity_vs_time.jpeg | thumb | center | 800 px | Fluorescence assay of bidirectional and unidirectional circuits without AIP-1 vs Time]] | ||
| Line 69: | Line 73: | ||
[[Image:IISER Kolkata--Bi-Intensity vs conc.jpeg | thumb | center | 800 px |Fluorescence assay of bidirectional and unidirectional circuits at different concentrations of AIP-1 at 5hrs ]] | [[Image:IISER Kolkata--Bi-Intensity vs conc.jpeg | thumb | center | 800 px |Fluorescence assay of bidirectional and unidirectional circuits at different concentrations of AIP-1 at 5hrs ]] | ||
| − | '''Final Comments:'''Thus we can conclude that our new part [https://parts.igem.org/Part:BBa_K3799057 BBa_K3799057] is indeed an improvement of the existing part [https://parts.igem.org/Part:BBa_K1022100 BBa_K1022100] in significantly decreasing the leaky gene expression under the P2 promoter | + | '''Final Comments:''' |
| + | |||
| + | Thus we can conclude that our new part [https://parts.igem.org/Part:BBa_K3799057 BBa_K3799057] is indeed an improvement of the existing part [https://parts.igem.org/Part:BBa_K1022100 BBa_K1022100] in significantly decreasing the leaky gene expression under the P2 promoter | ||
Latest revision as of 02:14, 22 October 2021
Bidirectional AIP-1 sensor
This is an improvement of the existing part BBa_K1022100 , by flipping the direction of p2 promoter to the opposite direction of pBAD promoter.
Usage and Biology
This is a high throughput sensing module that can sense the presence of AIP-I molecules. This part consists of a pBAD promoter, an AIP sensor infrastructure (BBa_I746101), an AIP inducible promoter P2 and a GFP reporter.
In the natural system, the signalling Auto-inducing peptide (termed AIP) is made from AgrD, while AgrB, a transmembrane protein that is responsible for the cyclization. AgrC and AgrA, form the two-component system responsible for AIP detection and response relay, respectively. Upon binding to AIP, AgrC, a transmembrane histidine kinase receptor, facilitates the phosphorylation of the transcriptional activator AgrA. The phosphorylated AgrA, in turn, activates gene expression regulated by P2 and P3 promoters. There are four known variants of AIP (AIP I-IV) with different molecular structures and cross-inhibitory activity.
This part has been improved from BBa_K1022100 by flipping the direction of p2 promoter to the opposite direction of pBAD promoter.
In the existing part (Unidirectional circuit), we observed a significant increase in GFP expression in samples with zero AIP concentration. Based on this observation, we inferred that this could be due to the leaky gene expression of GFP. We have improved the existing part in such a way that the leaky gene expression reduced significantly.
Design
To solve the leaky gene expression we designed a bidirectional circuit. We Used Hi-Fi DNA assembly kit to make this bidirectional circuit. First, we divided the existing part (BBa_K1022100) into three segments and ordered those from Twist Bioscience. These three fragments were assembled in two different ways to produce the existing part as well as our designed improved part. For these assemblies, we designed two different sets of primers (a total of eight primers) using the NEBilder tool. We extended our three gene fragments with appropriate primers to produce overlap sequences at both ends of each fragment.
To make the improved part, we designed the overlap sequences in such a way that the p2 promoter along with the GFP reporter is flipped. This will make the direction of pBAD promoter and P2 promoter opposite to each other, which will significantly decrease the leaky gene expression of the GFP reporter.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1105
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1493
Illegal BamHI site found at 1045
Illegal BamHI site found at 2377 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 295
Illegal AgeI site found at 720 - 1000COMPATIBLE WITH RFC[1000]
Characterization
Introduction
We observed that there is leaky gene expression in the Unidirectional circuit. We modified the existing part to reduce the leaky gene expression. By flipping the gene we expect that the leaky gene expression will be reduced. To check our hypotheses, we have compared the expression of GFP in the unidirectional and the bidirectional genetic circuit.
Experiment
First, we PCR extended the three DNA segments (pBAD-agrC, P2-GFP,agrA,) of the unidirectional circuit with specific primers. To confirm the success of PCR extension an Agarose Gel Electrophoresis was performed. As evident from the picture below, correct bands for all three amplified DNA segments were obtained. (Extended pBAD-agrC ~ 1.4 Kb, Extended P2-GFP ~ 1Kb, Extended agrA ~ 800bp and extended backbone~2kb.
After that, the same three segments (pBAD-agrC, P2-GFP, agrA, and psb1C3 plasmid backbone) were PCR amplified with different sets of specific primers. This extended overlap helps flip the P2-GFP fragments. From the gel electrophoresis image below. We can observe similar band sizes as above because the difference in length of the extension is very low.
After the successful extension of the segments, those extended fragments were assembled using Hi-Fi DNA assembly master mix. The reaction mixture was transformed in the E.Coil DH5a. After that, colony PCR of both the unidirectional and bidirectional circuit was performed using VF2 and VR primers to identify the bacterial colony with our desired gene circuits.
As evident from the colony PCR results, positive inserts for both unidirectional and bidirectional assemblies were obtained.
After the successful assembling of both the fragments, we planned to see the expression level of GFP in both bidirectional and unidirectional circuits. For that, first, the AIP-1 generator (BBa_K3799006) was expressed in 40ml of bacterial culture to obtain AIP-1 molecules. After expressing for 8-10 hours, the culture was centrifuged and the supernatant was filter-sterilized. The supernatant was lyophilized to concentrate the AIP-1 molecule. After full lyophilization, it was resuspended in 3 ml of NFW to make a stock solution of AIP1.
The stock solution of AIP-1 molecules was diluted to make solutions of different concentrations, from 0% to 100%, where 0% was the negative control and 100% was the one-third dilution of our stock solution.
The two types of cells were incubated (one with unidirectional plasmid and the other with bidirectional plasmid) at 30℃ for 8 hours without AIP-1.
The fluorescence intensity of those two types of cells in absence of AIP-1 molecules was measured at different time points and it was observed that there is a higher fluorescence intensity in cells with unidirectional circuits as compared to the cells with bidirectional circuits. This infers that the bidirectional circuit has less leaky gene expression than that of the unidirectional circuit.
We also wanted to check whether this difference in leaky gene expression is consistent with different concentrations of AIP-1.
To check this, we induced our two types of cells (one with unidirectional plasmid and the other with bidirectional plasmid) with different concentrations of the AIP-1 solution that we mentioned above. From our data, we infer that this leaky gene expression of the unidirectional circuit is consistent in different concentrations of AIP-1 molecules. Also, we can observe a slight decrease in the gap of expression of GFP as the concentration of AIP-1 increases. This may be because of the fact that the ratio of leaky gene expression and the AIP-1 induced expression decreases as the AIP-1 concentration increases.
Final Comments:
Thus we can conclude that our new part BBa_K3799057 is indeed an improvement of the existing part BBa_K1022100 in significantly decreasing the leaky gene expression under the P2 promoter