Difference between revisions of "Part:BBa K3725020"
Michellejing (Talk | contribs) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| − | |||
| − | + | <b>Overview</b> | |
| + | |||
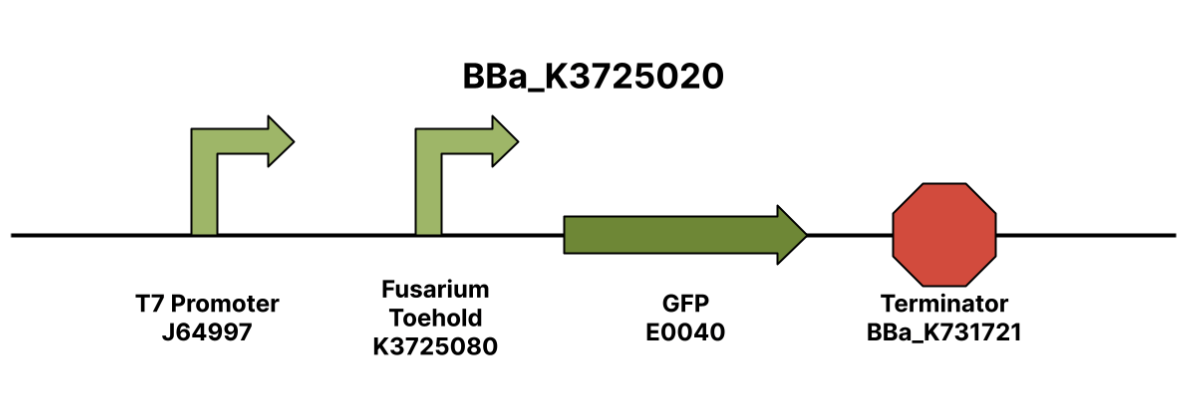
| + | The <i>F. oxysporum f.sp. lycopersici</i> toehold part is designed to be used in conjunction with the T7 <i>F. oxysporum f.sp. lycopersici</i> trigger (Part BBa_K3725070) in order to express GFP as a part of the engineered toehold switch system. The described structure was designed using NUPACK (Nucleic Acid Package) software’s Design feature. This was done by finding a unique gene of the <i>F. oxysporum</i> species that encodes for pathogenicity: the FRP1 gene. After inputting the FRP1 gene sequence into the NUPACK software using an input code given by Takashi et. al., pairs of trigger sequences and switch sequences were outputted. The trigger sequences given were 36 base-pair long sequences from the FRP1 gene, and the switch sequences given were reverse complementary to the trigger sequences. The pairs were ordered by normalized ensemble defect, so the pair with the lowest normalized ensemble defect was used. We ordered the insert in a pUCIDT Amp plasmid from Integrated DNA Technologies. | ||
| + | |||
| + | |||
| + | |||
| + | <strong>Description</strong> | ||
| + | |||
| + | Toehold biosensors, which are composed of a switch and trigger, are highly orthogonal riboregulators that activate translation in response to a specific RNA sequence. The switch is composed of a hairpin loop structure that represses translation through its complementary bases in between the ribosomal binding site and the start codon, which is followed by a linker sequence. Once the toehold is exposed to the trigger sequence, the complementary base pairs on the trigger will bind to the toehold, which exposes the ribosomal binding site. RNA polymerase can then bind to the RBS and initiate translation of the reporter protein. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <b>Design</b> | ||
| + | |||
| + | The construction of a disease-specific biosensor required us to find a gene unique to the pathogen. When the switch turns on and GFP is expressed, we can confirm that the specific pathogen is present. For the detection of <i>Fusarium oxysporum f. sp. lycopersici</i>, Lambert iGEM focused on the FRP1 gene. This gene was selected because it was required for pathogenicity and was unique to the species of interest. We obtained the sequence via UniProt, an online database of protein sequences. Lambert iGEM used the code from Takahashi et. al provided by Megan McSweeney from the Styczynski Lab at the Georgia Institute of Technology to design the switch and trigger sequences on NUPACK [2]. The team selected the pair from NUPACK with the lowest normalized ensemble defect (NED) to maximize the chances of successful compatibility [3]. Once we obtained the sequences for the toehold pair, we constructed the toehold and trigger via SnapGene. | ||
| + | |||
| + | |||
| + | [[File:Fusriumtoehold.png|thumb|center|500px|<i>Figure 1. BBa_K3725020 Fusarium Toehold w/ GFP Reporter Construct | ||
| + | </i>]] | ||
| + | |||
| + | <b>Experience</b> | ||
| + | |||
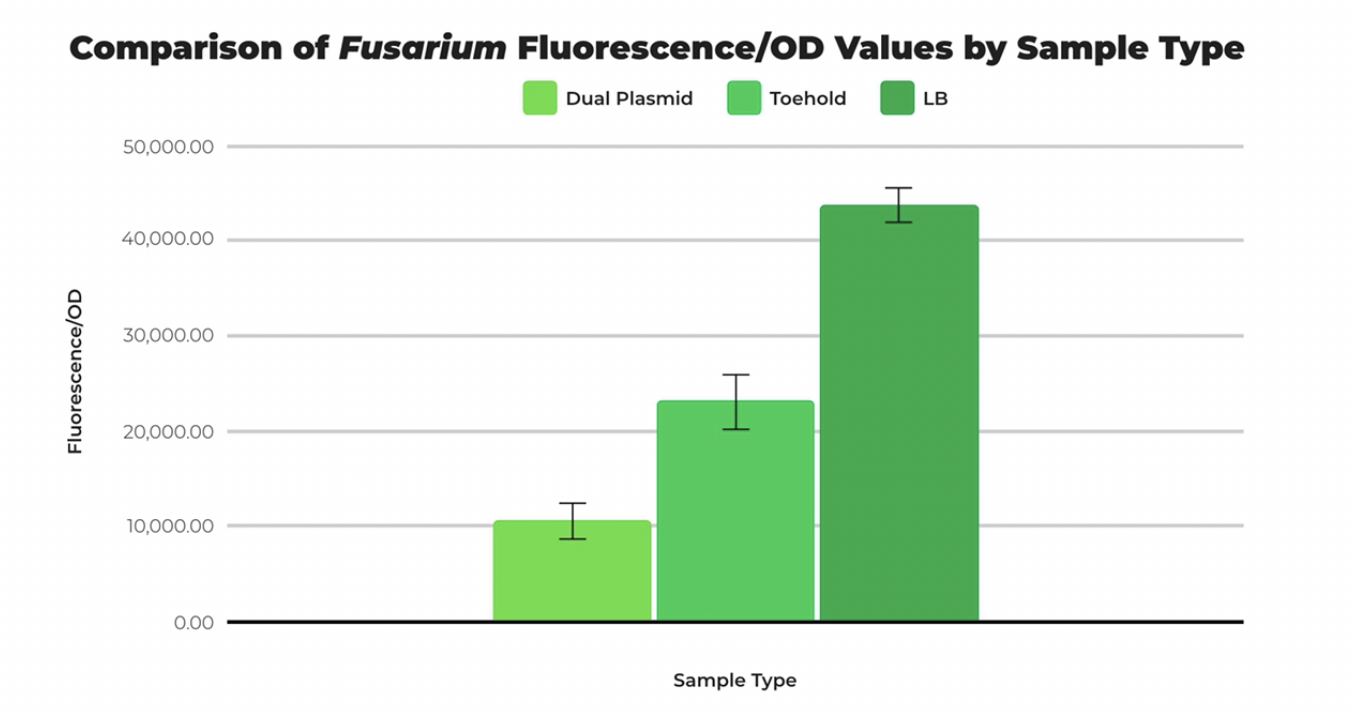
| + | The T7 Fusarium Trigger (Part BBa_K3725070) and the Fusarium Toehold w/ GFP Reporter (Part BBa_K3725020) are intended to be compatible with each other and be used in conjunction. To test the compatibility of the trigger sequence with the toehold sequence, a dual-plasmid transformation was performed, and fluorescence was measured in comparison with cells transformed with only the toehold part and pUC19 (positive control). All samples were divided by optical density to obtain a standardized unit. The fluorescence per optical density unit measurement of cells transformed with both the toehold and trigger sequences was not statistically greater in comparison to the measurements of cells transformed with only the toehold part and pUC19 plasmid. We concluded that there were a variety of factors affecting the compatibility including lack of IPTG and high ensemble normalized defect rate. | ||
| + | |||
| + | Therefore, Lambert iGEM decided to use a redesigned toehold switch sequence, Improved Fusarium Toehold with GFP Reporter BBa_K3725022, obtained from NUPACK, and performed dual plasmid transformation. The T7 Fusarium Trigger BBa_K3725070 binded more effectively to the Improved Toehold as shown in the data below. | ||
| + | |||
| + | |||
| + | [[File:Datata.png|thumb|center|500px|<i>Figure 2. Mean fluorescence/OD of Fusarium dual plasmid transformation compared to toehold and plain LB with SEM error bars. DP stands for dual plasmid, TH stands for toehold only. Ran at a gain of 60. | ||
| + | |||
| + | </i>]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
Latest revision as of 22:40, 21 October 2021
Overview
The F. oxysporum f.sp. lycopersici toehold part is designed to be used in conjunction with the T7 F. oxysporum f.sp. lycopersici trigger (Part BBa_K3725070) in order to express GFP as a part of the engineered toehold switch system. The described structure was designed using NUPACK (Nucleic Acid Package) software’s Design feature. This was done by finding a unique gene of the F. oxysporum species that encodes for pathogenicity: the FRP1 gene. After inputting the FRP1 gene sequence into the NUPACK software using an input code given by Takashi et. al., pairs of trigger sequences and switch sequences were outputted. The trigger sequences given were 36 base-pair long sequences from the FRP1 gene, and the switch sequences given were reverse complementary to the trigger sequences. The pairs were ordered by normalized ensemble defect, so the pair with the lowest normalized ensemble defect was used. We ordered the insert in a pUCIDT Amp plasmid from Integrated DNA Technologies.
Description
Toehold biosensors, which are composed of a switch and trigger, are highly orthogonal riboregulators that activate translation in response to a specific RNA sequence. The switch is composed of a hairpin loop structure that represses translation through its complementary bases in between the ribosomal binding site and the start codon, which is followed by a linker sequence. Once the toehold is exposed to the trigger sequence, the complementary base pairs on the trigger will bind to the toehold, which exposes the ribosomal binding site. RNA polymerase can then bind to the RBS and initiate translation of the reporter protein.
Design
The construction of a disease-specific biosensor required us to find a gene unique to the pathogen. When the switch turns on and GFP is expressed, we can confirm that the specific pathogen is present. For the detection of Fusarium oxysporum f. sp. lycopersici, Lambert iGEM focused on the FRP1 gene. This gene was selected because it was required for pathogenicity and was unique to the species of interest. We obtained the sequence via UniProt, an online database of protein sequences. Lambert iGEM used the code from Takahashi et. al provided by Megan McSweeney from the Styczynski Lab at the Georgia Institute of Technology to design the switch and trigger sequences on NUPACK [2]. The team selected the pair from NUPACK with the lowest normalized ensemble defect (NED) to maximize the chances of successful compatibility [3]. Once we obtained the sequences for the toehold pair, we constructed the toehold and trigger via SnapGene.
Experience
The T7 Fusarium Trigger (Part BBa_K3725070) and the Fusarium Toehold w/ GFP Reporter (Part BBa_K3725020) are intended to be compatible with each other and be used in conjunction. To test the compatibility of the trigger sequence with the toehold sequence, a dual-plasmid transformation was performed, and fluorescence was measured in comparison with cells transformed with only the toehold part and pUC19 (positive control). All samples were divided by optical density to obtain a standardized unit. The fluorescence per optical density unit measurement of cells transformed with both the toehold and trigger sequences was not statistically greater in comparison to the measurements of cells transformed with only the toehold part and pUC19 plasmid. We concluded that there were a variety of factors affecting the compatibility including lack of IPTG and high ensemble normalized defect rate.
Therefore, Lambert iGEM decided to use a redesigned toehold switch sequence, Improved Fusarium Toehold with GFP Reporter BBa_K3725022, obtained from NUPACK, and performed dual plasmid transformation. The T7 Fusarium Trigger BBa_K3725070 binded more effectively to the Improved Toehold as shown in the data below.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 784