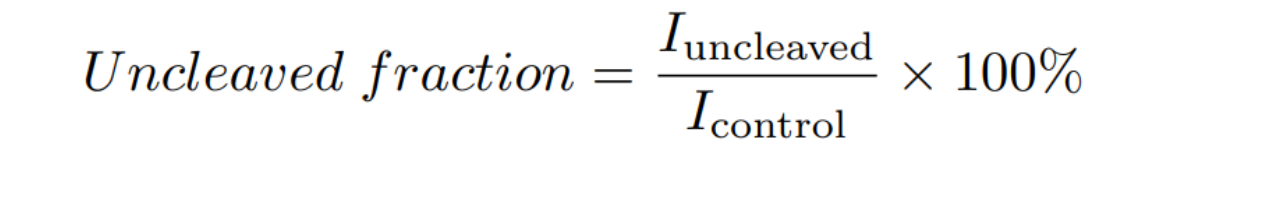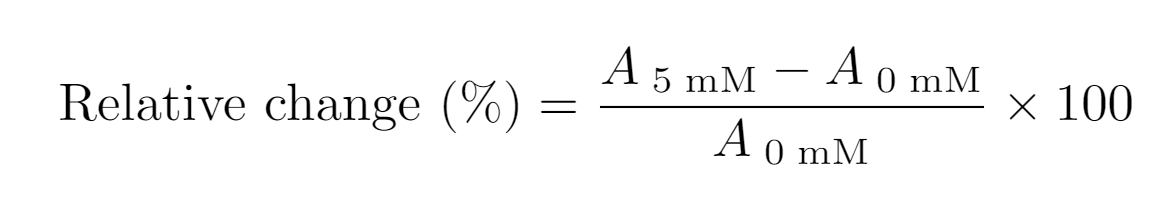Difference between revisions of "Part:BBa K3806016"
| (13 intermediate revisions by the same user not shown) | |||
| Line 10: | Line 10: | ||
| + | <html> | ||
| + | <div id="toc" class="toc"><div id="toctitle"><h2>Contents</h2></div> | ||
| + | <ul> | ||
| + | <li class="toclevel-1 tocsection-1"><a href="#Usage_and_Biology"><span class="tocnumber">1</span> <span class="toctext">Usage and Biology</span></a> | ||
| + | |||
| + | <ul> | ||
| + | <li class="toclevel-2 tocsection-3"><a href="#Cleavage characterization of the aptazyme-regulated genetic construct"><span class="tocnumber">1.1</span> <span class="toctext">Aptazyme-regulated gene expression mechanism</span></a></li> | ||
| + | |||
| + | </ul></li> | ||
| + | <li class="toclevel-1 tocsection-2"><a href="#Experimental_results"><span class="tocnumber">2</span> <span class="toctext">Experimental results</span></a> | ||
| + | <ul> | ||
| + | <li class="toclevel-2 tocsection-3"><a href="#Cleavage characterization of the aptazyme-regulated genetic construct"><span class="tocnumber">2.1</span> <span class="toctext">Cleavage characterization of the aptazyme-regulated genetic construct </span></a></li> | ||
| + | <li class="toclevel-2 tocsection-4"><a href="#Determination of expression profiles via absorbance | ||
| + | "><span class="tocnumber">2.2</span> <span class="toctext">Determination of expression profiles via absorbance | ||
| + | </span></a></li> | ||
| + | </ul> | ||
| + | </li> | ||
| + | <li class="toclevel-1 tocsection-5"><a href="#References"><span class="tocnumber">3</span> <span class="toctext">References</span></a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | </html> | ||
==Usage and Biology== | ==Usage and Biology== | ||
| − | Sensing small molecules is the foundation of many applications, ranging from disease diagnosis, prognosis, or treatment to detecting small pollutants in the environment. Synthetic genetic switches are a promising tool to detect and quantify small molecules. Conventional synthetic biology-based biosensors are based on transcription factors [1]. However, transcription factors are not easily reprogrammable with respect to ligand selectivity. Antibody-based biosensors can be easily developed into new sensing capabilities. Unfortunately, these types of sensors are not suitable to detect low molecular weight compounds [ | + | Sensing small molecules is the foundation of many applications, ranging from disease diagnosis, prognosis, or treatment to detecting small pollutants in the environment. Synthetic genetic switches are a promising tool to detect and quantify small molecules. Conventional synthetic biology-based biosensors are based on transcription factors [1]. However, transcription factors are not easily reprogrammable with respect to ligand selectivity. Antibody-based biosensors can be easily developed into new sensing capabilities. Unfortunately, these types of sensors are not suitable to detect low molecular weight compounds [2]. RNA-based biosensors are an interesting alternative since they present a remarkable flexibility to be engineered into sensing a wide range of analytes with high sensitivity and selectivity. In particular, aptazymes, ligand regulated self-cleaving ribozymes, are of special interest as cleavage of the aptazyme can be coupled to gene expression <i>in vivo</i> or <i>in vitro</i>. Moreover, newly developed methods such as DRIVER (<i>de novo</i> rapid <i>in vitro</i> evolution of RNA biosensors) enable rapid, automated, and multiplexed engineering of aptazymes sequences to diverse ligands [3]. |
| − | The TU Delft 2021 team provides the iGEM community with a novel genetic switch construct (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank"><b>BBa_K3806016</b></a></HTML>) in which an aptazyme sequence is fused to the <i>lacZ</i> reporter gene to convert | + | The TU Delft 2021 team provides the iGEM community with a novel genetic switch construct (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank"><b>BBa_K3806016</b></a></HTML>) in which an aptazyme sequence is fused to the <i>lacZ</i> reporter gene to convert ligand concentration to a colorimetric read-out. The <HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank"><b>BBa_K3806016</b></a></HTML> contains a theophylline-binding aptazyme sequence [3], yet this part is designed to be modular and serve as a template to engineer other ligand-specific genetic circuits by swapping the aptazyme domain. This part was successfully expressed in a cell-free system, and its biosensing performance was compared to <HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank"><b>BBa_K3806014</b></a></HTML>, showing an improvement in the dynamic range and time-response capabilities. |
<HTML><h3>Aptazyme-regulated gene expression mechanism</h3></html> | <HTML><h3>Aptazyme-regulated gene expression mechanism</h3></html> | ||
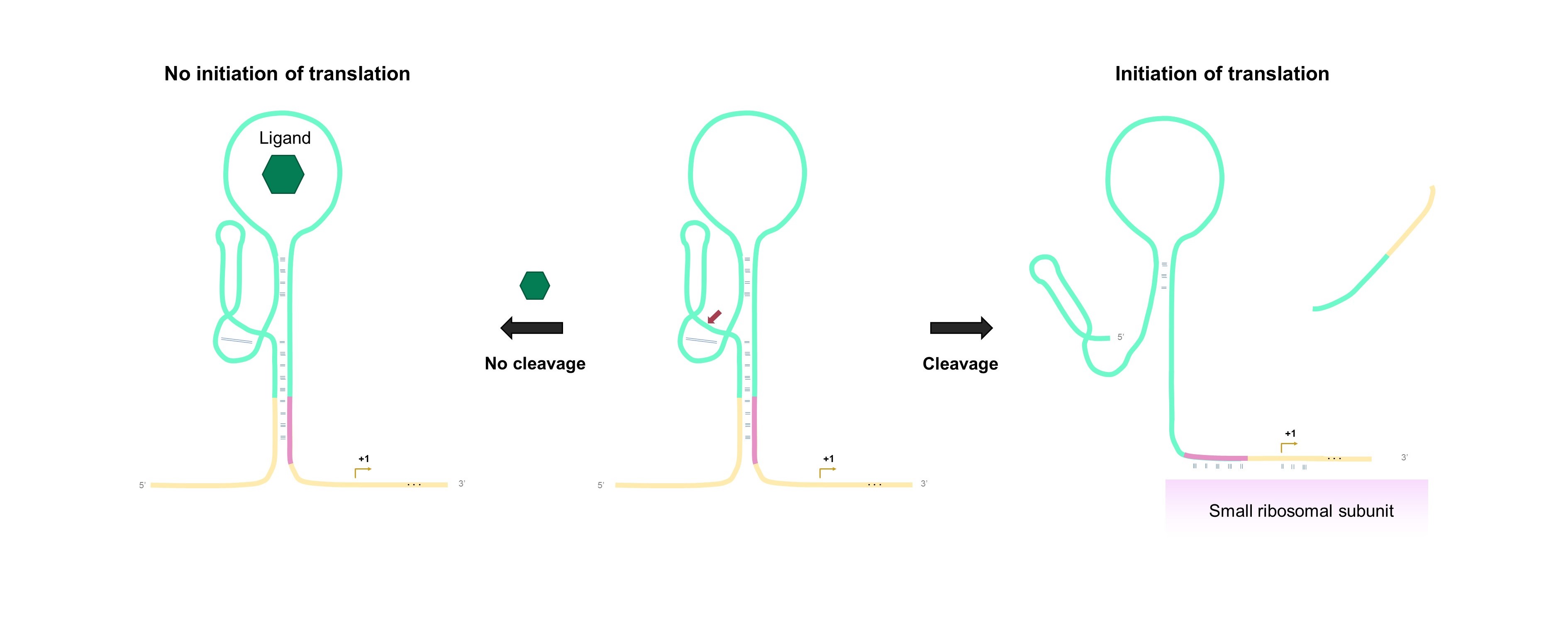
| − | The aptazyme-regulated expression of <i>lacZ</i> depends on the accessibility of the ribosomal binding site (RBS) for translation initiation as inspired by the study of Klauser & Hartig [ | + | The aptazyme-regulated expression of <i>lacZ</i> depends on the accessibility of the ribosomal binding site (RBS) for translation initiation as inspired by the study of Klauser & Hartig [4]. After transcription of the DNA template comprising the aptazyme and fused <i>lacZ</i> reporter gene, a part of the RBS is hidden in the stem of the aptazyme. When the aptazyme is stabilized upon binding of the ligand, the RBS remains partly sequestered by its antisense strand, and translation is mostly prohibited. Cleavage of the aptazyme in the absence of the ligand liberates the RBS (Fig. 1). As a result, the ribosome can bind to the RBS and translate the downstream reporter gene to the β-galactosidase protein. Subsequently, the β-galactosidase enzyme can catalyze the colorimetric conversion of enzymatic substrates such as chlorophenol red-b-D-galactopyranoside (CPRG) and X-gal. |
| − | [[File:T--TUDelft--Mech parts semicRBS.jpg| | + | [[File:T--TUDelft--Mech parts semicRBS.jpg|700px|center|]] |
| − | <html><b | + | <html><b>Fig. 1 Aptazyme-regulated gene expression mechanism.</b></html> Binding of the ligand renders a catalytically inactive aptazyme, the RBS remains partly sequestered by its antisense strand, repressing translation (left). In the absence of the ligand, self-cleavage of the aptazyme frees the RBS, resulting in the binding of the small ribosomal subunit and initiation of translation (right). |
==Experimental results== | ==Experimental results== | ||
| Line 31: | Line 52: | ||
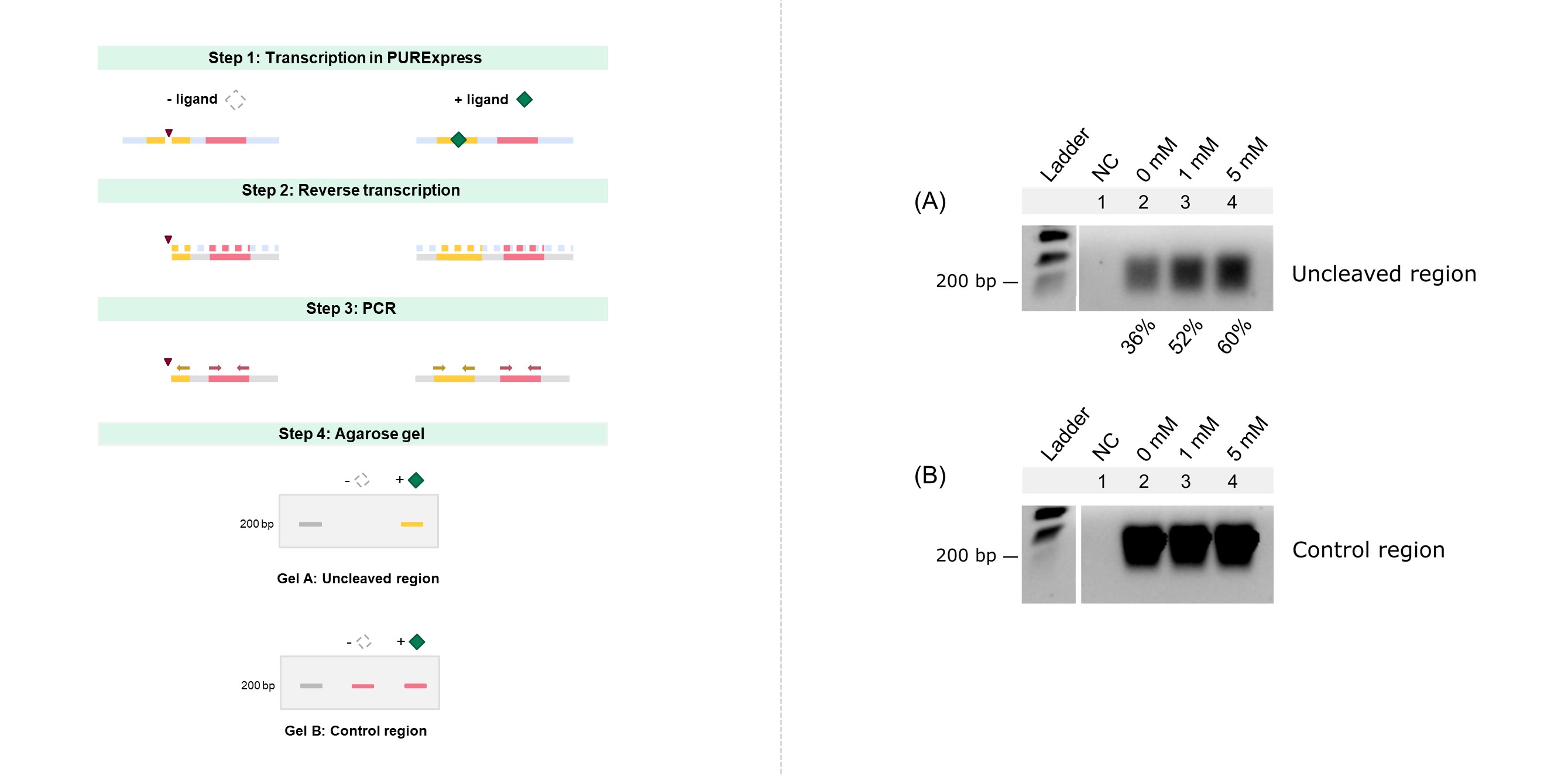
[[File:T--TUDelft--RTqPCR semicRBS.jpg|800px|center|]] | [[File:T--TUDelft--RTqPCR semicRBS.jpg|800px|center|]] | ||
| − | <html | + | <html><b>Fig. 2 Scheme of the 4 steps of the cleavage characterization procedure (left). Agarose gel analysis of the theophylline-dependent cleavage activity of the aptazyme after expression in PURExpress (right). </b></html> (A) PCR product after amplification of the uncleaved region (the uncleaved fraction is displayed in the bottom of the gel). (B) PCR product after amplification of the control region. Lanes: (Ladder) dsDNA ladder. (1) NC: negative control without DNA template. (2) 0 mM theophylline (3) 1 mM theophylline. (4) 5 mM theophylline. |
| Line 45: | Line 66: | ||
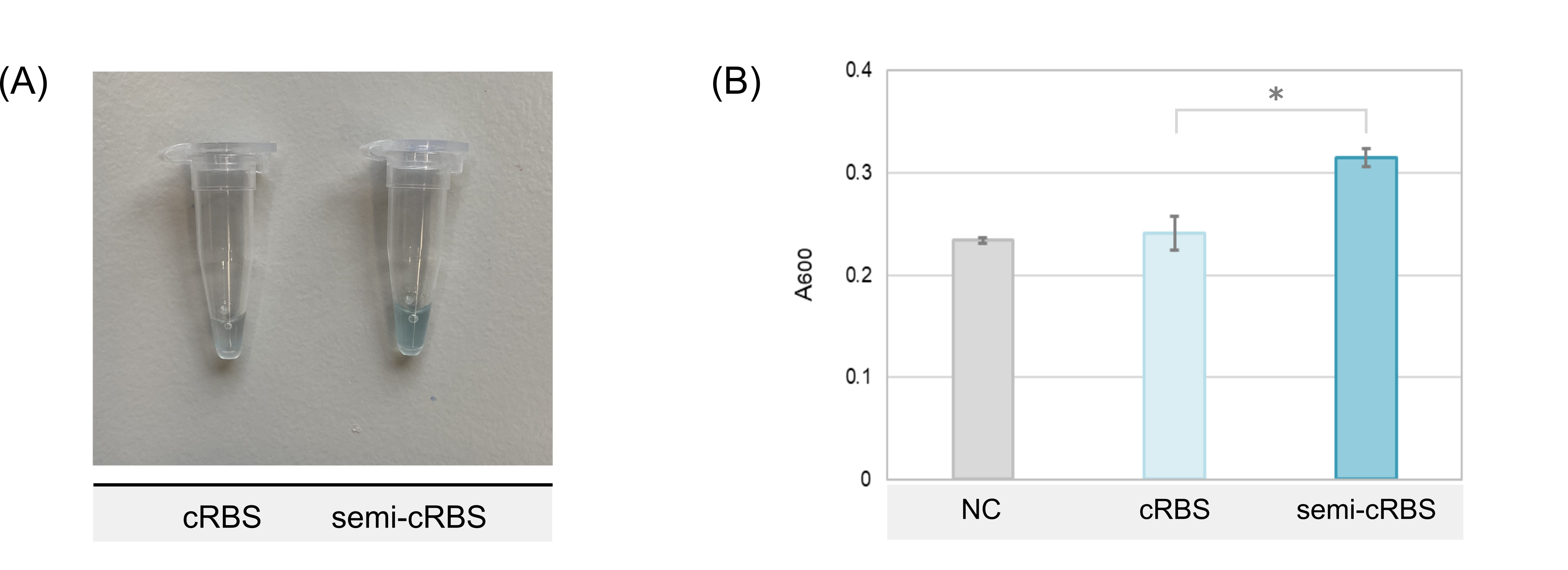
[[File:T--TUDelft--X-gal-cRBS-semicRBS parts.png|500px|center|]] | [[File:T--TUDelft--X-gal-cRBS-semicRBS parts.png|500px|center|]] | ||
| − | <html | + | <html><b>Fig. 3 Comparison of X-gal production levels between the cRBS (BBa_K3806014) and semi-cRBS (BBa_K3806016) constructs in the absence of ligand.</b></html>(A) Image of the PUREfrex2.0 reaction after 2 hours of expression at 37 °C, and incubation with X-gal at room temperature overnight (one of the replicates for each design is shown in this picture). (B) Absorbance measurement at 600 nm of the X-gal production. Data represents the mean ± SE (n = 3). The asterisk indicates a p-value ˂ 0.05 in Student's t-test. |
As expected, the expression level of the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) part as measured by absorbance is significantly higher than cRBS (<html><a href="https://parts.igem.org/Part:BBa_K3806014">BBa_K3806014</a>).</html> | As expected, the expression level of the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) part as measured by absorbance is significantly higher than cRBS (<html><a href="https://parts.igem.org/Part:BBa_K3806014">BBa_K3806014</a>).</html> | ||
| − | + | <html><br><br><br></html> | |
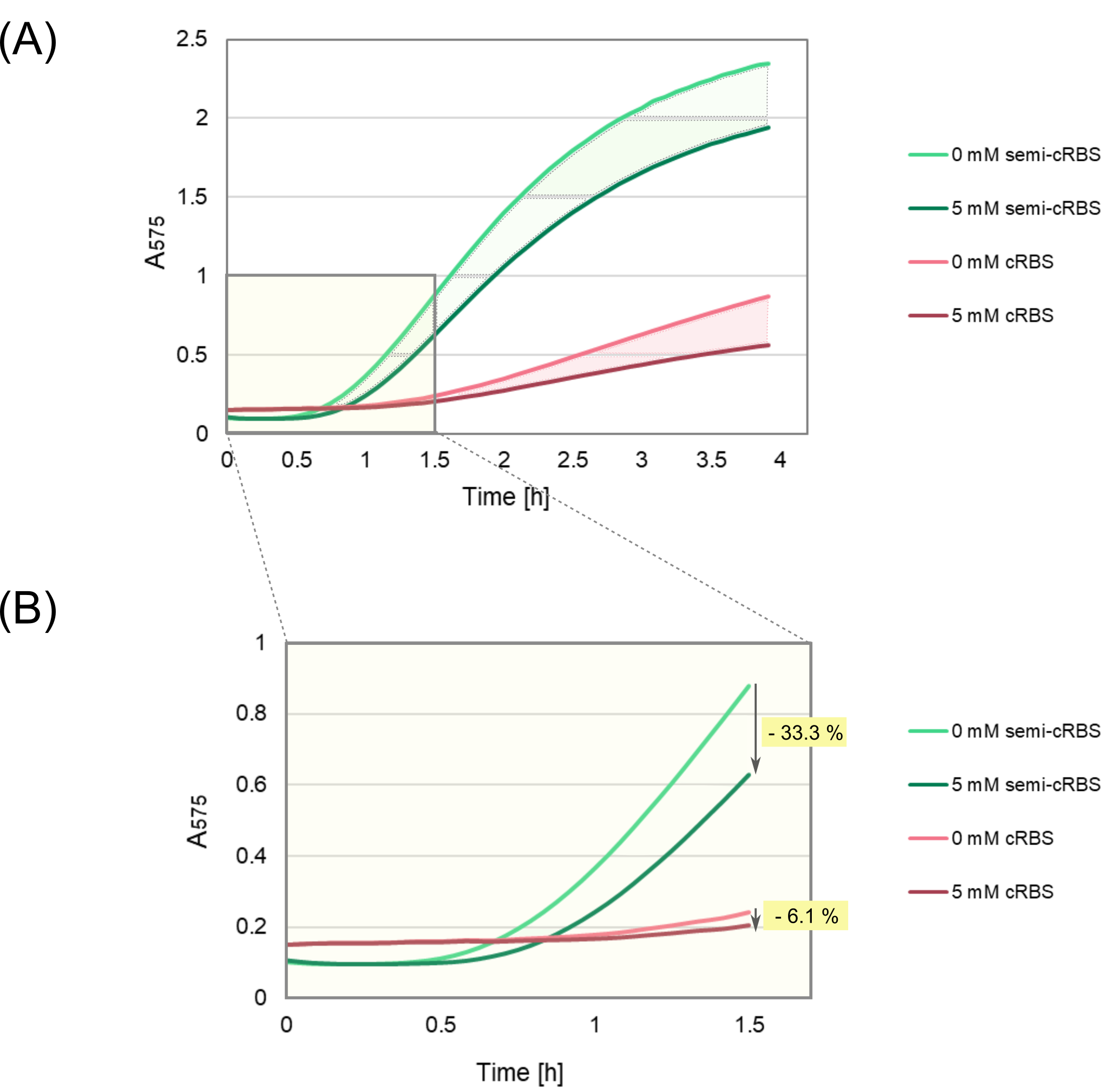
To further determine the theophylline impact on the color change induced by the aptazyme-based biosensors, the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) part was expressed in PURExpress in the presence and absence of theophylline. For colorimetric reactions to occur, the enzymatic substrate CPRG was supplied to the cell-free system reactions. The expression profile of this part was again compared to cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) to examine their performance as biosensors. The product formation was determined by measuring the absorbance at 575 nm (Fig. 4). | To further determine the theophylline impact on the color change induced by the aptazyme-based biosensors, the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) part was expressed in PURExpress in the presence and absence of theophylline. For colorimetric reactions to occur, the enzymatic substrate CPRG was supplied to the cell-free system reactions. The expression profile of this part was again compared to cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) to examine their performance as biosensors. The product formation was determined by measuring the absorbance at 575 nm (Fig. 4). | ||
[[File:T--TUDelft--cRBS-semi-cRBS-abs.png|500px|center|]] | [[File:T--TUDelft--cRBS-semi-cRBS-abs.png|500px|center|]] | ||
| − | <html | + | <html><b>Fig. 4</i> Comparison of the colorimetric response between the semi-cRBS (BBa_K3806016) and cRBS (BBa_K3806014) designs.</b></html> CPR production was quantified as a measure of absorbance at 575 nm. (A) During 4 hours of expression. The area enclosed between the absorbance curves corresponding to 0 and 5 mM theophylline is shaded in pink and green for the cRBS and semi-cRBS, respectively. (B) During the first 1.5 hours of expression. The decrease in expression due to theophylline addition is indicated with an arrow. |
| − | As expected, the presence of theophylline results in reduced expression compared to the absence of theophylline in both | + | As expected, the presence of theophylline results in reduced expression compared to the absence of theophylline in both designs. Expression profiles after 4 hours of expression indicated that the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) was better expressed than the cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) construct (Fig. 4). As an indication of both (i) <b>the dynamic range</b> represented by the absorbance difference between the smallest and highest ligand concentration (A<sub>0mM</sub> - A<sub>5mM</sub>), and (ii) <b>the response time</b> after 4 hours of expression, the area enclosed between the absorbance curves corresponding to 0 and 5 mM theophylline was calculated for both cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) and semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>). For the cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) design, the calculated area was 0.40 h x absorbance units (Au), whereas for the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) the area was 1.03 h x Au, meaning a 2.57-fold increase. This indicates a larger dynamic range and faster response time of the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>) part. In addition, we determined the decrease in the expression level caused by the addition of 5 mM theophylline after 1.5 hours of expression as: |
[[File:T--TUDelft--Relative-change.png|400px|center|]] | [[File:T--TUDelft--Relative-change.png|400px|center|]] | ||
| Line 62: | Line 83: | ||
The semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a><HTML/>) already shows a 33.3% decrease of expression after 1.5 hour, in contrast to the 6.1% decrease in the cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) design, which confirms the improved response time of the system when using the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>). | The semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a><HTML/>) already shows a 33.3% decrease of expression after 1.5 hour, in contrast to the 6.1% decrease in the cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>) design, which confirms the improved response time of the system when using the semi-cRBS (<HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>). | ||
| − | Although, <html><a href="https://2021.igem.org/Team:TUDelft/Results">alternative experiments</a></html> using a control part without an aptazyme sequence showed inhibitory effects of theophylline on the PURE cell-free system, the expression profiles and the cleavage characterization of <HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>, support that the <i>lacZ</i> expression from this part is ligand-responsive. Moreover, this part has shown to be an improved version of | + | Although, <html><a href="https://2021.igem.org/Team:TUDelft/Results">alternative experiments</a></html> using a control part without an aptazyme sequence showed inhibitory effects of theophylline on the PURE cell-free system, the expression profiles and the cleavage characterization of <HTML><a href="https://parts.igem.org/Part:BBa_K3806016" target="_blank">BBa_K3806016</a></HTML>, support that the <i>lacZ</i> expression from this part is ligand-responsive. Moreover, this part has shown to be an improved version of <HTML><a href="https://parts.igem.org/Part:BBa_K3806014" target="_blank">BBa_K3806014</a></HTML>. |
==References== | ==References== | ||
Latest revision as of 22:00, 21 October 2021
Theophylline-binding aptazyme regulating lacZ expression (semi-cRBS). With T7 promoter.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3204
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 2321
- 1000COMPATIBLE WITH RFC[1000]
Contents
Usage and Biology
Sensing small molecules is the foundation of many applications, ranging from disease diagnosis, prognosis, or treatment to detecting small pollutants in the environment. Synthetic genetic switches are a promising tool to detect and quantify small molecules. Conventional synthetic biology-based biosensors are based on transcription factors [1]. However, transcription factors are not easily reprogrammable with respect to ligand selectivity. Antibody-based biosensors can be easily developed into new sensing capabilities. Unfortunately, these types of sensors are not suitable to detect low molecular weight compounds [2]. RNA-based biosensors are an interesting alternative since they present a remarkable flexibility to be engineered into sensing a wide range of analytes with high sensitivity and selectivity. In particular, aptazymes, ligand regulated self-cleaving ribozymes, are of special interest as cleavage of the aptazyme can be coupled to gene expression in vivo or in vitro. Moreover, newly developed methods such as DRIVER (de novo rapid in vitro evolution of RNA biosensors) enable rapid, automated, and multiplexed engineering of aptazymes sequences to diverse ligands [3].
The TU Delft 2021 team provides the iGEM community with a novel genetic switch construct (BBa_K3806016) in which an aptazyme sequence is fused to the lacZ reporter gene to convert ligand concentration to a colorimetric read-out. The BBa_K3806016 contains a theophylline-binding aptazyme sequence [3], yet this part is designed to be modular and serve as a template to engineer other ligand-specific genetic circuits by swapping the aptazyme domain. This part was successfully expressed in a cell-free system, and its biosensing performance was compared to BBa_K3806014, showing an improvement in the dynamic range and time-response capabilities.
Aptazyme-regulated gene expression mechanism
The aptazyme-regulated expression of lacZ depends on the accessibility of the ribosomal binding site (RBS) for translation initiation as inspired by the study of Klauser & Hartig [4]. After transcription of the DNA template comprising the aptazyme and fused lacZ reporter gene, a part of the RBS is hidden in the stem of the aptazyme. When the aptazyme is stabilized upon binding of the ligand, the RBS remains partly sequestered by its antisense strand, and translation is mostly prohibited. Cleavage of the aptazyme in the absence of the ligand liberates the RBS (Fig. 1). As a result, the ribosome can bind to the RBS and translate the downstream reporter gene to the β-galactosidase protein. Subsequently, the β-galactosidase enzyme can catalyze the colorimetric conversion of enzymatic substrates such as chlorophenol red-b-D-galactopyranoside (CPRG) and X-gal.
Fig. 1 Aptazyme-regulated gene expression mechanism. Binding of the ligand renders a catalytically inactive aptazyme, the RBS remains partly sequestered by its antisense strand, repressing translation (left). In the absence of the ligand, self-cleavage of the aptazyme frees the RBS, resulting in the binding of the small ribosomal subunit and initiation of translation (right).
Experimental results
Cleavage characterization of the aptazyme-regulated genetic construct
To assess the ligand-dependent cleavage activity of the theophylline aptazyme in BBa_K3806016, the aptazyme-fused lacZ transcript product was analysed by RT-PCR. First the genetic construct was expressed in PURExpress for 1 hour (Fig. 2, step 1) followed by a reverse transcription (RT) reaction to convert the transcribed mRNA to cDNA (step 2). These cDNA products were then amplified using two primer sets. One that flanks the cleavage site (uncleaved region) and one that flanks a region downstream the cleavage site (control region). In the former case, it should be noted that only the uncleaved mRNA fraction will be PCR amplified (step 3). As a final step, the PCR product was visualized on an agarose gel (step 4). A lower band intensity is expected with decreasing concentration of theophylline, meaning that aptazyme self-cleaved and the forward primer flanking the cleavage site was unable to bind to the 5’ of the cDNA to produce a PCR product. The region downstream the cleavage point was used as control for transcription, RT, PCR and loading in the agarose gel, as band intensity should be similar between samples regardless of theophylline addition.Fig. 2 Scheme of the 4 steps of the cleavage characterization procedure (left). Agarose gel analysis of the theophylline-dependent cleavage activity of the aptazyme after expression in PURExpress (right). (A) PCR product after amplification of the uncleaved region (the uncleaved fraction is displayed in the bottom of the gel). (B) PCR product after amplification of the control region. Lanes: (Ladder) dsDNA ladder. (1) NC: negative control without DNA template. (2) 0 mM theophylline (3) 1 mM theophylline. (4) 5 mM theophylline.
ImageJ was used to quantify the intensity (I) of the gel bands, and the uncleaved fraction was determined by:
The computed uncleaved fractions were 36%, 52%, and 60% for 0, 1 and 5 mM theophylline, respectively (Fig. 2A). From this it can be concluded that the aptazyme presents theophylline-dependent cleavage activity in the PURExpress cell-free system, and that the fusion of the lacZ gene to the aptazyme sequence does not hinder aptazyme self-cleavage.
Determination of expression profiles via absorbance
To get a first insight on the performance of BBa_K3806016 (semi-cRBS), this part was expressed using a PURE based cell-free system (PUREfrex2.0) and X-gal as substrate. Theophylline was omitted from the reaction as this gives the maximum expression level of this construct. The expression level was also compared to BBa_K3806014 (cRBS), a related part in which the entire RBS is sequestered. It was hypothesized that BBa_K3806016 would present a higher expression because of an easier detachment of the antisense strand after aptazyme cleavage, which potentially hinders translation in BBa_K3806014. To confirm this, the product formation after 2 hours of expression at 37 °C, and incubation with X-gal at room temperature overnight.measured at 600 nm (Fig. 3).Fig. 3 Comparison of X-gal production levels between the cRBS (BBa_K3806014) and semi-cRBS (BBa_K3806016) constructs in the absence of ligand.(A) Image of the PUREfrex2.0 reaction after 2 hours of expression at 37 °C, and incubation with X-gal at room temperature overnight (one of the replicates for each design is shown in this picture). (B) Absorbance measurement at 600 nm of the X-gal production. Data represents the mean ± SE (n = 3). The asterisk indicates a p-value ˂ 0.05 in Student's t-test.
As expected, the expression level of the semi-cRBS (BBa_K3806016) part as measured by absorbance is significantly higher than cRBS (BBa_K3806014).
To further determine the theophylline impact on the color change induced by the aptazyme-based biosensors, the semi-cRBS (BBa_K3806016) part was expressed in PURExpress in the presence and absence of theophylline. For colorimetric reactions to occur, the enzymatic substrate CPRG was supplied to the cell-free system reactions. The expression profile of this part was again compared to cRBS (BBa_K3806014) to examine their performance as biosensors. The product formation was determined by measuring the absorbance at 575 nm (Fig. 4).
Fig. 4 Comparison of the colorimetric response between the semi-cRBS (BBa_K3806016) and cRBS (BBa_K3806014) designs. CPR production was quantified as a measure of absorbance at 575 nm. (A) During 4 hours of expression. The area enclosed between the absorbance curves corresponding to 0 and 5 mM theophylline is shaded in pink and green for the cRBS and semi-cRBS, respectively. (B) During the first 1.5 hours of expression. The decrease in expression due to theophylline addition is indicated with an arrow.
As expected, the presence of theophylline results in reduced expression compared to the absence of theophylline in both designs. Expression profiles after 4 hours of expression indicated that the semi-cRBS (BBa_K3806016) was better expressed than the cRBS (BBa_K3806014) construct (Fig. 4). As an indication of both (i) the dynamic range represented by the absorbance difference between the smallest and highest ligand concentration (A0mM - A5mM), and (ii) the response time after 4 hours of expression, the area enclosed between the absorbance curves corresponding to 0 and 5 mM theophylline was calculated for both cRBS (BBa_K3806014) and semi-cRBS (BBa_K3806016). For the cRBS (BBa_K3806014) design, the calculated area was 0.40 h x absorbance units (Au), whereas for the semi-cRBS (BBa_K3806016) the area was 1.03 h x Au, meaning a 2.57-fold increase. This indicates a larger dynamic range and faster response time of the semi-cRBS (BBa_K3806016) part. In addition, we determined the decrease in the expression level caused by the addition of 5 mM theophylline after 1.5 hours of expression as:
The semi-cRBS (BBa_K3806016
) already shows a 33.3% decrease of expression after 1.5 hour, in contrast to the 6.1% decrease in the cRBS (BBa_K3806014) design, which confirms the improved response time of the system when using the semi-cRBS (BBa_K3806016).Although, alternative experiments using a control part without an aptazyme sequence showed inhibitory effects of theophylline on the PURE cell-free system, the expression profiles and the cleavage characterization of BBa_K3806016, support that the lacZ expression from this part is ligand-responsive. Moreover, this part has shown to be an improved version of BBa_K3806014.
References
- [1] Weinberg, B. H., Pham, N., Caraballo, L. D., Lozanoski, T., Engel, A., Bhatia, S., & Wong, W. W. (2017). Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nature biotechnology, 35(5), 453–462.
- [2] Byrne, B., Stack, E., Gilmartin, N., & O'Kennedy, R. (2009). Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors (Basel, Switzerland), 9(6), 4407–4445.
- [3]Townshend, B., Xiang, J. S., Manzanarez, G., Hayden, E. J. and Smolke, C. (2021). A multiplexed, automated evolution pipeline enables scalable discovery and characterization of biosensors. Nat Commun, 12, 1437.
- [4] Klauser, B., & Hartig, J. S. (2013). An engineered small RNA-mediated genetic switch based on a ribozyme expression platform. Nucleic acids research, 41(10), 5542-5552.