Difference between revisions of "Part:BBa K3725040"
| (8 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K3725040 short</partinfo> | <partinfo>BBa_K3725040 short</partinfo> | ||
| + | |||
<b>Overview</b> | <b>Overview</b> | ||
| + | The T7 Phytophthora Trigger is designed to be used in conjunction with the Phytophthora Toehold w/ GFP Reporter (BBa_K3725010) to induce GFP expression for Lambert iGEM’s <i>P. Cryptogea</i> toehold biosensor. When the trigger RNA sequence is present, it binds to the complementary sequence in the toehold switch and unravels the hairpin loop allowing the reporter protein (GFP) to be expressed, producing green fluorescence. The sequence was designed via NUPACK using an input code provided by Takahashi et. al. We ordered the insert in a pUCIDT Kan plasmid from Integrated DNA Technologies. | ||
<strong>Description</strong> | <strong>Description</strong> | ||
| Line 14: | Line 16: | ||
<b>Design</b> | <b>Design</b> | ||
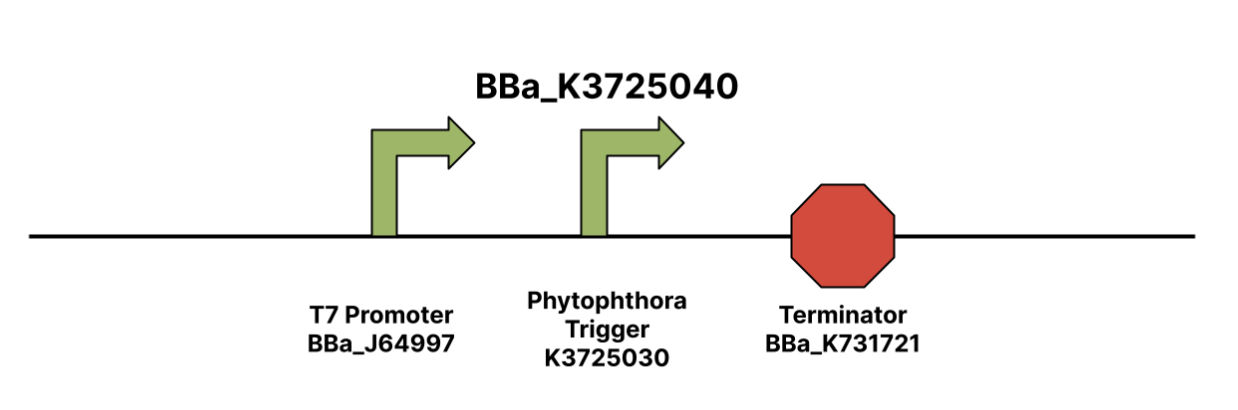
| − | The construction of a disease-specific biosensor required us to find a gene unique to the pathogen. When the switch turns on and GFP is expressed, we can confirm that the specific pathogen is present. For the detection of Phytophthora cryptogea, Lambert iGEM focused on the X24 gene. This gene was selected because it was required for pathogenicity and was unique to the species of interest. Biosafety note, the trigger sequence is not the full transcript sequence and therefore poses limited biosafety. We obtained the sequence via UniProt, an online database of protein sequences. Lambert iGEM used the code from Takahashi et. al provided by Megan McSweeney from the Styczynski Lab at the Georgia Institute of Technology to design the switch and trigger sequences on NUPACK. The team selected the pair from NUPACK with the lowest normalized ensemble defect (NED) to maximize the chances of successful compatibility. Once we obtained the sequences for the toehold pair, we constructed the toehold and trigger via SnapGene. | + | The construction of a disease-specific biosensor required us to find a gene unique to the pathogen. When the switch turns on and GFP is expressed, we can confirm that the specific pathogen is present. For the detection of <i>Phytophthora cryptogea</i>, Lambert iGEM focused on the X24 gene. This gene was selected because it was required for pathogenicity and was unique to the species of interest. Biosafety note, the trigger sequence is not the full transcript sequence and therefore poses limited biosafety. We obtained the sequence via UniProt, an online database of protein sequences. Lambert iGEM used the code from Takahashi et. al provided by Megan McSweeney from the Styczynski Lab at the Georgia Institute of Technology to design the switch and trigger sequences on NUPACK. The team selected the pair from NUPACK with the lowest normalized ensemble defect (NED) to maximize the chances of successful compatibility. Once we obtained the sequences for the toehold pair, we constructed the toehold and trigger via SnapGene. |
| − | + | [[File:T--Lambert GA--phytotrigger.png|thumb|center|500px|<i>Figure 1. BBa_K3725040 T7 Phytophthora Trigger Construct</i>]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| + | <b>Experience</b> | ||
| + | |||
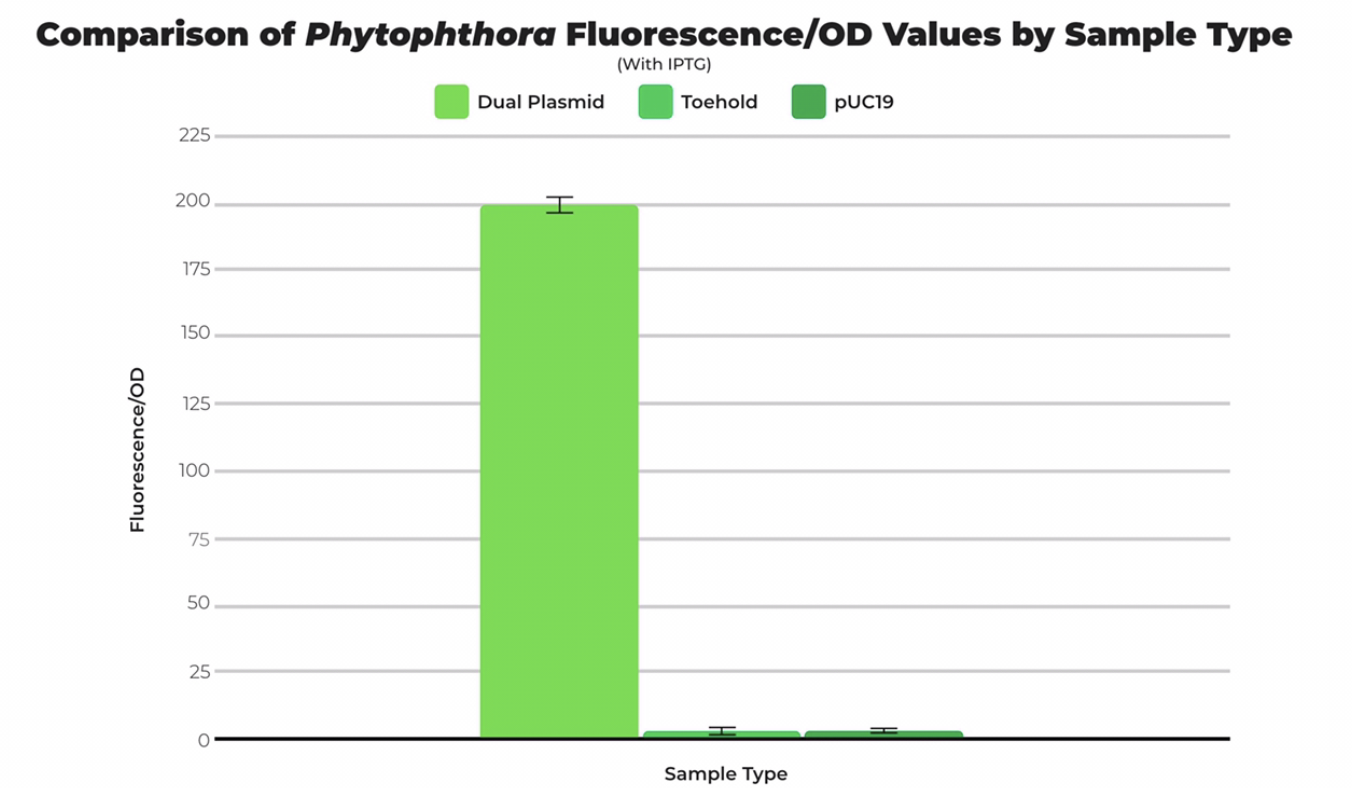
| + | The T7 Phytophthora Trigger (Part BBa_K3725040) and the Phytophthora Toehold w/ GFP Reporter (Part BBa_K3725010) are intended to be compatible with each other and used in conjunction. To test the compatibility of the trigger sequence with the toehold sequence, a dual-plasmid transformation was performed, and fluorescence was measured in comparison with cells transformed with only the toehold part and pUC19 (positive control). All samples were divided by optical density to obtain a standardized unit. The fluorescence per optical density unit measurement of cells transformed with both the toehold and trigger sequences was greater and had a statistically significant difference in comparison to cells transformed with only the toehold part and pUC19 plasmid | ||
| + | [[File:Phytodata.png|thumb|center|500px|<i>Figure 2. Mean fluorescence/OD of IPTG-induced Phytophthora dual plasmid transformation compared to toehold and pUC19 with SEM error bars. DP stands for dual plasmid, TH stands for toehold only. Ran at gain of 60.</i>]] | ||
| Line 29: | Line 31: | ||
<!-- --> | <!-- --> | ||
| − | <span class='h3bb'> | + | <span class='h3bb'></span> |
<partinfo>BBa_K3725040 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3725040 SequenceAndFeatures</partinfo> | ||
| Line 36: | Line 38: | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K3725040 parameters</partinfo> | <partinfo>BBa_K3725040 parameters</partinfo> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- --> | <!-- --> | ||
Latest revision as of 21:34, 21 October 2021
T7 Fusarium Trigger
Overview
The T7 Phytophthora Trigger is designed to be used in conjunction with the Phytophthora Toehold w/ GFP Reporter (BBa_K3725010) to induce GFP expression for Lambert iGEM’s P. Cryptogea toehold biosensor. When the trigger RNA sequence is present, it binds to the complementary sequence in the toehold switch and unravels the hairpin loop allowing the reporter protein (GFP) to be expressed, producing green fluorescence. The sequence was designed via NUPACK using an input code provided by Takahashi et. al. We ordered the insert in a pUCIDT Kan plasmid from Integrated DNA Technologies.
Description
Toehold biosensors, which are composed of a switch and trigger, are highly orthogonal riboregulators that activate translation in response to a specific RNA sequence. The switch is composed of a hairpin loop structure that represses translation through its complementary bases in between the ribosomal binding site and the start codon, which is followed by a linker sequence. Once the toehold is exposed to the trigger sequence, the complementary base pairs on the trigger will bind to the toehold, which exposes the ribosomal binding site. RNA polymerase can then bind to the RBS and initiate translation of the reporter protein.
Design
The construction of a disease-specific biosensor required us to find a gene unique to the pathogen. When the switch turns on and GFP is expressed, we can confirm that the specific pathogen is present. For the detection of Phytophthora cryptogea, Lambert iGEM focused on the X24 gene. This gene was selected because it was required for pathogenicity and was unique to the species of interest. Biosafety note, the trigger sequence is not the full transcript sequence and therefore poses limited biosafety. We obtained the sequence via UniProt, an online database of protein sequences. Lambert iGEM used the code from Takahashi et. al provided by Megan McSweeney from the Styczynski Lab at the Georgia Institute of Technology to design the switch and trigger sequences on NUPACK. The team selected the pair from NUPACK with the lowest normalized ensemble defect (NED) to maximize the chances of successful compatibility. Once we obtained the sequences for the toehold pair, we constructed the toehold and trigger via SnapGene.
Experience
The T7 Phytophthora Trigger (Part BBa_K3725040) and the Phytophthora Toehold w/ GFP Reporter (Part BBa_K3725010) are intended to be compatible with each other and used in conjunction. To test the compatibility of the trigger sequence with the toehold sequence, a dual-plasmid transformation was performed, and fluorescence was measured in comparison with cells transformed with only the toehold part and pUC19 (positive control). All samples were divided by optical density to obtain a standardized unit. The fluorescence per optical density unit measurement of cells transformed with both the toehold and trigger sequences was greater and had a statistically significant difference in comparison to cells transformed with only the toehold part and pUC19 plasmid
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]