Difference between revisions of "Part:BBa K4090006"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K4090006 short</partinfo> | <partinfo>BBa_K4090006 short</partinfo> | ||
| − | + | ==Introduction== | |
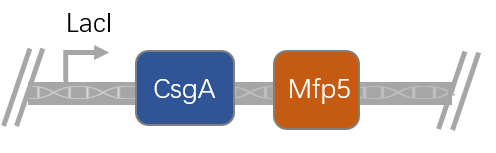
| − | + | CsgA is one of the several major components of E.coli biofilm. When it is co-expressed with the gene, it could bring the protein that the gene encodes to the cell surface. Mfp5 is found in mussel foot. It could induce the precipitation in vitro in the 1.5×SBF solution. This biobrick could induce the precipitation of hydroxyapatite. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K4090006 SequenceAndFeatures</partinfo> | <partinfo>BBa_K4090006 SequenceAndFeatures</partinfo> | ||
| + | ==Usage and Viability== | ||
| + | We designed the part as below. | ||
| + | [[File:T--SDSZ_China--csgA2.png|700px|thumb|center|Fig.1]] | ||
| + | <br><br> | ||
| + | After constructing the plasmids by Gibson Assembly, our team used PCR to test whether the plasmids were successfully constructed. Since we omitted a part of the sequence of CsgA at first, we inserted this part of gene later and used the gel electrophoresis to detect this part. The number of bp was exactly the number we expected, proving that the construction was successful. | ||
| + | [[File:T--SDSZ_China--csgA1.jpeg|500px|thumb|center|Fig.2]] | ||
| + | <br><br> | ||
| + | After proving the success of construction, electron diffraction was used to verify the function of the part in inducing the precipitation. The electron-diffraction diagram below showed the existence of crystal in the sample. Since there was no other crystals in the sample, it could prove the precipitation of the hydroxyapatite, which indirectly verified the ability of the biobrick in inducing the precipitation of hydroxyapatite. | ||
| + | [[File:T--SDSZ_China--electron diffraction-diagram.jpeg|500px|thumb|center|Fig.3]] | ||
| + | |||
==References== | ==References== | ||
Latest revision as of 19:11, 21 October 2021
csgA-Mfp5
Introduction
CsgA is one of the several major components of E.coli biofilm. When it is co-expressed with the gene, it could bring the protein that the gene encodes to the cell surface. Mfp5 is found in mussel foot. It could induce the precipitation in vitro in the 1.5×SBF solution. This biobrick could induce the precipitation of hydroxyapatite. Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 371
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 371
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 556
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 371
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 371
- 1000COMPATIBLE WITH RFC[1000]
Usage and Viability
We designed the part as below.
After constructing the plasmids by Gibson Assembly, our team used PCR to test whether the plasmids were successfully constructed. Since we omitted a part of the sequence of CsgA at first, we inserted this part of gene later and used the gel electrophoresis to detect this part. The number of bp was exactly the number we expected, proving that the construction was successful.
After proving the success of construction, electron diffraction was used to verify the function of the part in inducing the precipitation. The electron-diffraction diagram below showed the existence of crystal in the sample. Since there was no other crystals in the sample, it could prove the precipitation of the hydroxyapatite, which indirectly verified the ability of the biobrick in inducing the precipitation of hydroxyapatite.
References
[1] DHAMI N K, REDDY M S, MUKHERJEE A. Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites[J]. Journal of Microbiology and Biotechnology, 2013, 23(5): 707-714.
[2] OKWADHA G D, LI J. Optimum conditions for microbial carbonate precipitation[J]. Chemosphere, 2010, 81(9): 1143-1148. [16]LI W, LIU L P, ZHOU P P, et al. Calcite precipitation induced by bacteria and bacterially produced carbonic anhydrase[J]. Current Science, 2011, 100( 4) : 502 - 508.
[3] ANBU P,KANG C H,SHIN Y J, et al. Formations of calcium carbonate minerals by bacteria and its multiple applications[J]. Springerplus, 2016, 5(1): 1 – 26.
[4] Hale, L. V., Ma, Y. F. & Santerre, R. F. Semi-quantitative fuorescence analysis of calcein binding as a measurement of in vitro mineralization. Calcif. Tissue Int. 67, 80–84 (2000).Ramachandran S K.
[5] Dick J, Windt W D, Graef B D, et al. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species[J]. Biodegradation, 2016(4): 357-367.
[6] Qian C X, Chen H C, Ren L F, et al. Self-healing of early age cracks in cement-based materials by mineralization of carbonic anhydrase microorganism[J]. Frontiers in Microbiology, 2015 (6): 1-9.