Difference between revisions of "Part:BBa K3887006"
(→References) |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3887006 short</partinfo> | <partinfo>BBa_K3887006 short</partinfo> | ||
| − | + | Fre-L3-RebH is an fusion enzyme of Fre and RebH using linker L3. | |
| + | ===Description=== | ||
| + | RebH is a type of 7-halogenase enzyme used also during conversion of indole and used to biosynthesis other types of indigo using Trp, similar to SttH producing Tyrian Purple<sup>[1]</sup>. It is applied with Fre-L3-halogenasee fusion technology to increase solulbility<sup>[1,2]</sup>. Below shows the plasmid we constructed for Fre-L3-RebH. | ||
| + | [[File:T--BJU_China--Fig.1 RebH1.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center>Figure 1. Gene circuit of Fre-L3-RebH</center></b></p> | ||
| + | |||
| + | ===Experiments and Results=== | ||
| + | We constructed halogenase plasmids using Gibson Assembly and verified by colony PCR and gene sequencing . According to the results in Figure 3, we can roughly identify Fre-L3-RebH gene band obviously comparing with DNA Marker. And then the PCR product fragment was sent for sequencing and the results were also consistent with expected. | ||
| + | [[File:T--BJU_China--Fig.2 RebH2.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center>Figure 2. Molecular identification of Fre-L3-RebH</center></b></p> | ||
| + | Next, we started the protein level experiment to test gene expression. For better expression of enzymes, we optimize the induction condition of gene expression: three different temperatures (18℃, 30℃, 37℃) and inducer concentrations (0 mM, 0.1mM, 1mM) were set for test.the collected cells after induction were disrupted, both of the pellet and supernatant were used for SDS-PAGE detection (Figure 3-4). | ||
| + | From the results of three SDS-PAGE electrophoresis gels, it can be seen that compared with the control group induced by 0mM IPTG, there are obvious changes in the lanes induced by IPTG, which proves Fre-L3-RebH has been effectively expressed under the induction. | ||
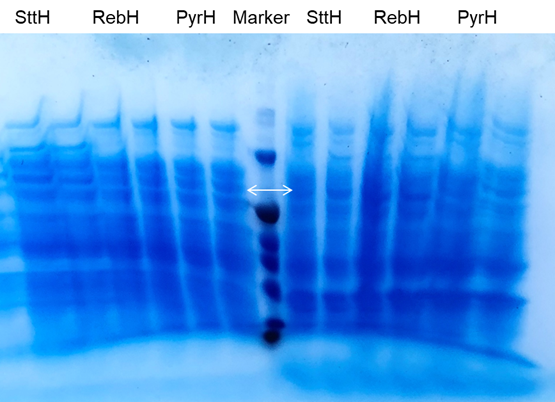
| + | [[File:PyrH3.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center> Figure 3. SDS-PAGE Gel of Fre-L3-RebH/PyrH(Left is supernatant, Right is precipitation) </center></b></p> | ||
| + | [[File:T--BJU_China--Fig.4 RebH4.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center> Figure 4. SDS-PAGE Gel of Fre-L3-RebH(Left is supernatant, Right is precipitation) </center></b></p> | ||
| + | Through the expression of Fre-L3-RebH at different temperatures under the same IPTG concentration, there is a significant decrease in RebH when induced at 18℃ compared with the expression levels at 30℃ and 37℃. Meanwhile, it is not difficult to see that the content of halogenase expressed in the supernatant solution is low. This may be due to low solublity of halogenases, even through Fre-L3 protein connection, the solubility is still not effectively improved, most enzymes are still exsit in the precipitation. Or it may also be related to our parameter settings in the cell disruption. Because of the insufficient ultrasonication strength, the protein is wrapped in cytoplasm then forms precipitate. | ||
| + | Subsequently, we performed the protein expression induction experiment again with optimized induction conditions and ultrasonication strength, and better results were obtained (Figure 5). | ||
| + | [[File:T--BJU_China--Fig.5 RebH-PyrH5.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center> Figure 5. SDS-PAGE Gel of three halogenases(Left is supernatant, Right is precipitation) </center></b></p> | ||
| + | Finally, we did a fermentation experiment with E.coli BL21 containing Fre-L3-RebH and the results were shown in Figure 8, from which we can see different indigo derivatives as expected. | ||
| + | [[File:T--BJU_China--Fig.6 RebH6.png|600px|thumb|center]] | ||
| + | <p class="figure-description"><b><center> Figure 6. Fermentation Results of Fre-L3-RebH (Left is supernatant, Right is precipitation) </center></b></p> | ||
| + | |||
| + | ===References=== | ||
| + | |||
| + | [1]Lee, J., Kim, J., Song, J.E. et al. Production of Tyrian purple indigoid dye from tryptophan in Escherichia coli. Nat Chem Biol 17, 104–112 (2021) | ||
| + | |||
| + | [2]Alfieri A, Malito E, Orru R, Fraaije MW, Mattevi A. Revealing the moonlighting role of NADP in the structure of a flavin-containing monooxygenase. Proc Natl Acad Sci U S A. 2008 May 6;105(18):6572-7 | ||
| + | |||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 17:50, 21 October 2021
Fre-L3-RebH
Fre-L3-RebH is an fusion enzyme of Fre and RebH using linker L3.
Description
RebH is a type of 7-halogenase enzyme used also during conversion of indole and used to biosynthesis other types of indigo using Trp, similar to SttH producing Tyrian Purple[1]. It is applied with Fre-L3-halogenasee fusion technology to increase solulbility[1,2]. Below shows the plasmid we constructed for Fre-L3-RebH.
Experiments and Results
We constructed halogenase plasmids using Gibson Assembly and verified by colony PCR and gene sequencing . According to the results in Figure 3, we can roughly identify Fre-L3-RebH gene band obviously comparing with DNA Marker. And then the PCR product fragment was sent for sequencing and the results were also consistent with expected.
Next, we started the protein level experiment to test gene expression. For better expression of enzymes, we optimize the induction condition of gene expression: three different temperatures (18℃, 30℃, 37℃) and inducer concentrations (0 mM, 0.1mM, 1mM) were set for test.the collected cells after induction were disrupted, both of the pellet and supernatant were used for SDS-PAGE detection (Figure 3-4). From the results of three SDS-PAGE electrophoresis gels, it can be seen that compared with the control group induced by 0mM IPTG, there are obvious changes in the lanes induced by IPTG, which proves Fre-L3-RebH has been effectively expressed under the induction.
Through the expression of Fre-L3-RebH at different temperatures under the same IPTG concentration, there is a significant decrease in RebH when induced at 18℃ compared with the expression levels at 30℃ and 37℃. Meanwhile, it is not difficult to see that the content of halogenase expressed in the supernatant solution is low. This may be due to low solublity of halogenases, even through Fre-L3 protein connection, the solubility is still not effectively improved, most enzymes are still exsit in the precipitation. Or it may also be related to our parameter settings in the cell disruption. Because of the insufficient ultrasonication strength, the protein is wrapped in cytoplasm then forms precipitate. Subsequently, we performed the protein expression induction experiment again with optimized induction conditions and ultrasonication strength, and better results were obtained (Figure 5).
Finally, we did a fermentation experiment with E.coli BL21 containing Fre-L3-RebH and the results were shown in Figure 8, from which we can see different indigo derivatives as expected.
References
[1]Lee, J., Kim, J., Song, J.E. et al. Production of Tyrian purple indigoid dye from tryptophan in Escherichia coli. Nat Chem Biol 17, 104–112 (2021)
[2]Alfieri A, Malito E, Orru R, Fraaije MW, Mattevi A. Revealing the moonlighting role of NADP in the structure of a flavin-containing monooxygenase. Proc Natl Acad Sci U S A. 2008 May 6;105(18):6572-7
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1709
Illegal XhoI site found at 1623
Illegal XhoI site found at 2269 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 2155
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1690
Illegal BsaI.rc site found at 2272