Difference between revisions of "Part:BBa K3983003"
| (5 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3983003 short</partinfo> | <partinfo>BBa_K3983003 short</partinfo> | ||
| − | + | ||
=== Profile === | === Profile === | ||
| − | ==== Name: efeB-amilGFP ==== | + | ==== Name: pro-efeB-amilGFP ==== |
| − | ==== Base Pairs: | + | |
| + | ==== Base Pairs: 2064 bp ==== | ||
==== Origin: Escherichia coli str. K-12 substr. MG1655 ==== | ==== Origin: Escherichia coli str. K-12 substr. MG1655 ==== | ||
| − | ==== Properties: | + | ==== Properties: an antioxidant enzyme system ==== |
=== Usage and Biology === | === Usage and Biology === | ||
| Line 40: | Line 41: | ||
==== Base Pairs: 2107 bp ==== | ==== Base Pairs: 2107 bp ==== | ||
==== Origin: Escherichia coli str. K-12 substr. MG1655 ==== | ==== Origin: Escherichia coli str. K-12 substr. MG1655 ==== | ||
| − | ==== Properties: | + | ==== Properties: an antioxidant enzyme system ==== |
=== Experimental approach === | === Experimental approach === | ||
| Line 48: | Line 49: | ||
This step is used to test if the plasmids efeB-amilGFP extracted from the E. coli DH5α are successful and could be used to do double enzyme digestion later in the process. Channel2-8: efeB-amilGFP succeeded | This step is used to test if the plasmids efeB-amilGFP extracted from the E. coli DH5α are successful and could be used to do double enzyme digestion later in the process. Channel2-8: efeB-amilGFP succeeded | ||
| + | |||
| + | == Proof of function == | ||
=== The growth of engineering bacteria under H2O2 treatment conditions === | === The growth of engineering bacteria under H2O2 treatment conditions === | ||
[[File:T--The Webb Schools--BBa K3983003-figure4.jpg|500px|thumb|center|Figure 4. Line chart of the OD600 of bacteria A, C and D against hours in 2.5mM H2O2...]] | [[File:T--The Webb Schools--BBa K3983003-figure4.jpg|500px|thumb|center|Figure 4. Line chart of the OD600 of bacteria A, C and D against hours in 2.5mM H2O2...]] | ||
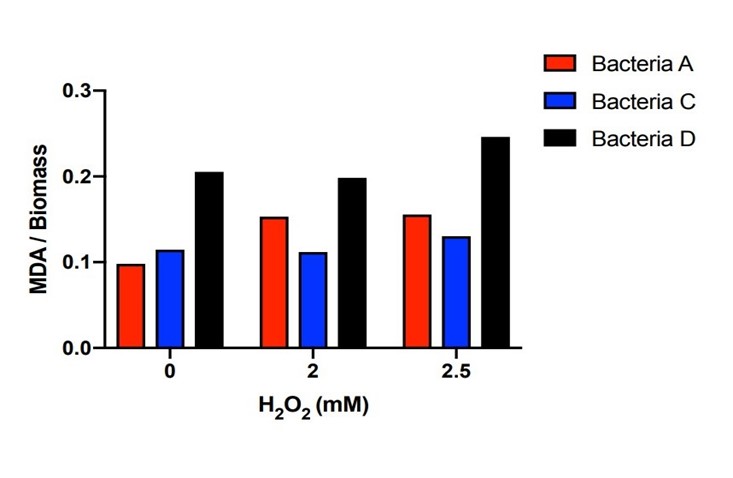
| − | The bacteria A contains plasmid A, pUC57-efeB. The bacteria C contains plasmid C, pUC57-efeB-amilGFP. The bacteria D contains plasmid D, pUC57. The bacteria are placed in 2.5mM hydrogen peroxide and their growth is measured for the first 4 hours. Bacteria D, without the efeB gene, has lower growth than bacteria A and bacteria C. This shows that efeB gene helps bacteria to grow when ROS is present. | + | The bacteria A contains plasmid A, pUC57-efeB. The bacteria C contains plasmid C, pUC57-efeB-amilGFP. The bacteria D contains plasmid D, pUC57. The bacteria are placed in 2.5mM hydrogen peroxide and their growth is measured for the first 4 hours. Bacteria D, without the efeB gene, has lower growth than bacteria A and bacteria C. This shows that efeB gene helps bacteria to grow when ROS is present. |
=== MDA and Fluorescence Measuring === | === MDA and Fluorescence Measuring === | ||
| Line 101: | Line 104: | ||
| − | |||
| − | |||
| − | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K3983003 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3983003 SequenceAndFeatures</partinfo> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 17:04, 21 October 2021
pro-efeB-amilGFP
Profile
Name: pro-efeB-amilGFP
Base Pairs: 2064 bp
Origin: Escherichia coli str. K-12 substr. MG1655
Properties: an antioxidant enzyme system
Usage and Biology
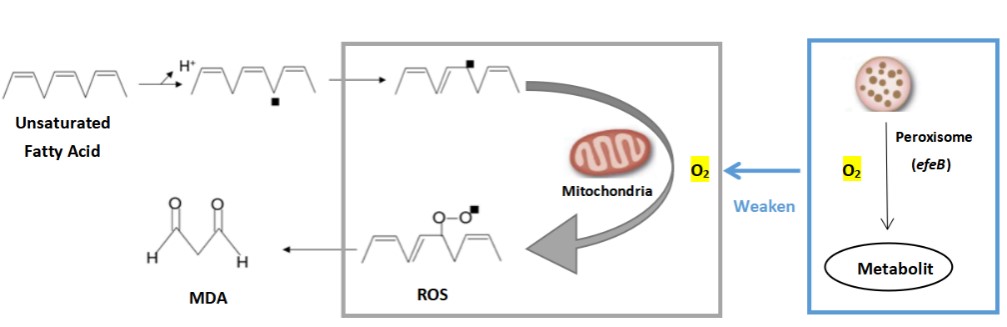
In modern society, more and more people suffer from mild or severe depression. It has been reported that malondialdehyde (MDA) level in the plasma of depressed patients is significantly increased. After receiving conventional antidepressant treatment, the patient's MDA level decreased to the same as that of healthy people. Therefore, researchers believe that oxidative stress may play an important role in the occurrence and development of depression, and the activity of antidepressant therapy may be mediated by improving oxidative stress/antioxidant function. We attempt to express an antioxidant enzyme system (peroxidase gene efeB) in the cell or on the surface of the engineered probiotic bacteria to inhibit the production of malondialdehyde (MDA) by human cells and prevent cell oxidation from causing health damage to the body. So as to prevent or alleviate the condition of depression. The oxidation rate of peroxisome increases in proportion to the increase of oxygen tension. Especially in the case of high oxygen concentrations, the oxidation reaction of peroxisomes dominates. This characteristic allows peroxisomes to protect cells from the toxic effects of high concentrations of oxygen. efeB reduces the oxidative stress in the cell by competing with mitochondria for oxygen, and reduces the MDA produced during the oxidation of lipids.
Construct design
Peroxidase EfeB, originally studied as a substrate of the E. coli "double arginine" translocation system, is the first recognized peroxidase for bacterial dye decolorization. Subsequent studies have shown that EfeB is a peroxidase with heme as a prosthetic group, and has a specific iron transport function.We use E. coli as the starting strain and construct an engineered strain of efeB to explore the effects of efeB on bacterial growth, malondialdehyde concentration, ROS synthesis and antioxidant pathways under different oxidative stress conditions.
In the process of cultivating microorganisms, namely Escherichia coli, because of their small size, they cannot be directly observed with the naked eye, and a certain method is needed for monitoring. In cell biology and molecular biology, the green fluorescent protein (GFP) gene is often used as a reporter gene. Through genetic engineering technology, the green fluorescent protein (GFP) gene can be transferred into the genomes of different species and continue to be expressed in offspring. . Therefore, we constructed the amilGFP engineered bacteria to show the growth status (number and vitality) of E. coli by observing the strength of the green fluorescent protein signal.
The T7 promoter is often used for protein overexpression. It is powerful and specific. It is completely controlled by T7 RNAP. When T7 RNAP is present in the cell, the T7 expression system occupies an absolute advantage compared to the host expression system. Its expression The speed is 5 times that of the former.
BBa_K592010
Name: amilGFP
Base Pairs: 699bp
Origin: Acropora millepora
Properties: A yellow chromoprotein
Usage and Biology
This part is useful as a reporter and it naturally exhibits strong yellow color when expressed.
BBa_K3983002
Name: pro-efeB
Base Pairs: 2107 bp
Origin: Escherichia coli str. K-12 substr. MG1655
Properties: an antioxidant enzyme system
Experimental approach
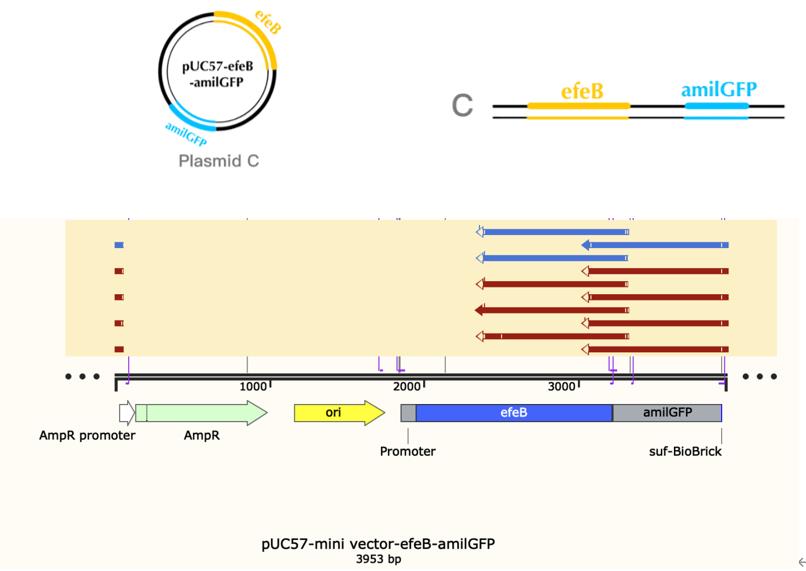
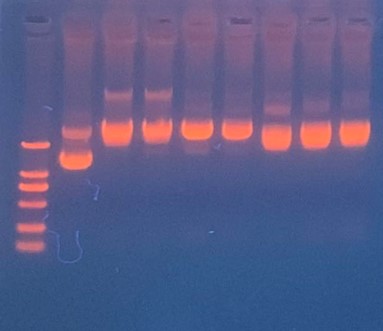
Production, purification, and sequcing analysis of recombinant efeB-amilGFP
This step is used to test if the plasmids efeB-amilGFP extracted from the E. coli DH5α are successful and could be used to do double enzyme digestion later in the process. Channel2-8: efeB-amilGFP succeeded
Proof of function
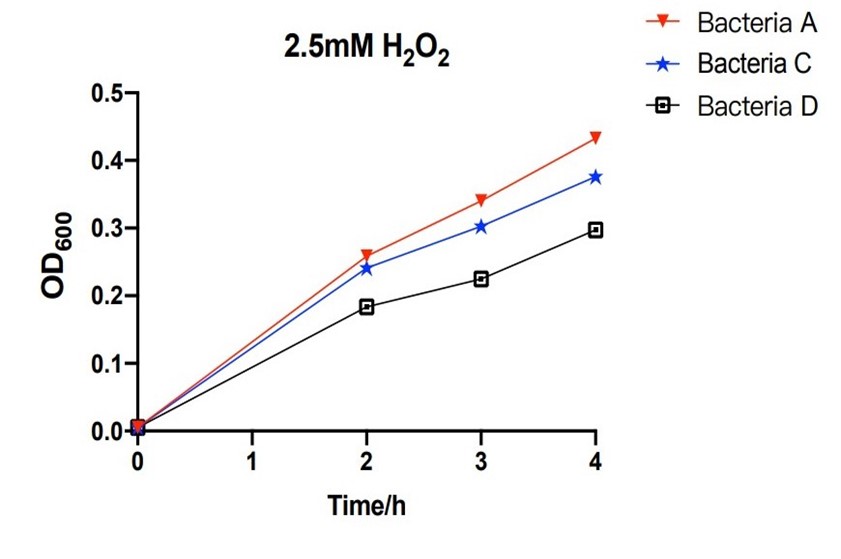
The growth of engineering bacteria under H2O2 treatment conditions
The bacteria A contains plasmid A, pUC57-efeB. The bacteria C contains plasmid C, pUC57-efeB-amilGFP. The bacteria D contains plasmid D, pUC57. The bacteria are placed in 2.5mM hydrogen peroxide and their growth is measured for the first 4 hours. Bacteria D, without the efeB gene, has lower growth than bacteria A and bacteria C. This shows that efeB gene helps bacteria to grow when ROS is present.
MDA and Fluorescence Measuring
Bacteria A, C, D were placed in hydrogen peroxide solution for 21 hours and the concentration of MDA was measured. Bacteria A and C kept the MDA concentration dramatically lower than those of Bacteria D under the hydrogen peroxide concentration of 0mM, 2mM and 2.5mM. There is a certain amount of MDA concentration within DH5α when the H2O2 concentration is 0mM. Starting from the original DH5α MDA concentration to the higher MDA concentration imposed by the 2mM and 2.5mM H2O2 concentration, Bacteria A and C with the efeB gene all effectively reduced the MDA concentration to the level lower than Bacteria D.
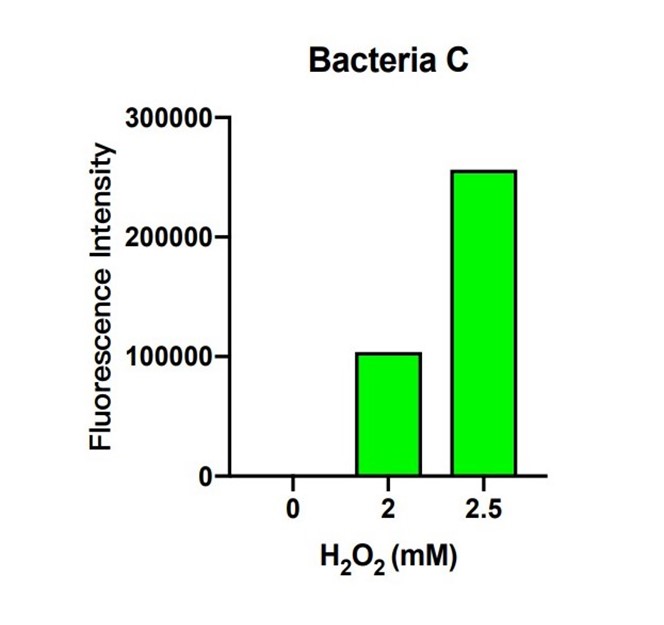
In bacteria C, the expression of GFP is controlled by the expression of efeB. The more intense the fluorescence is, the better efeB will express. H2O2 climbed from 0 to 2.5 mM, and the intensity of fluorescence climbed higher as well. The tendency of the intensity shows that the expression of efeB increases with the H2O2 concentration. Expression of efeB disturbs the production of MDA.
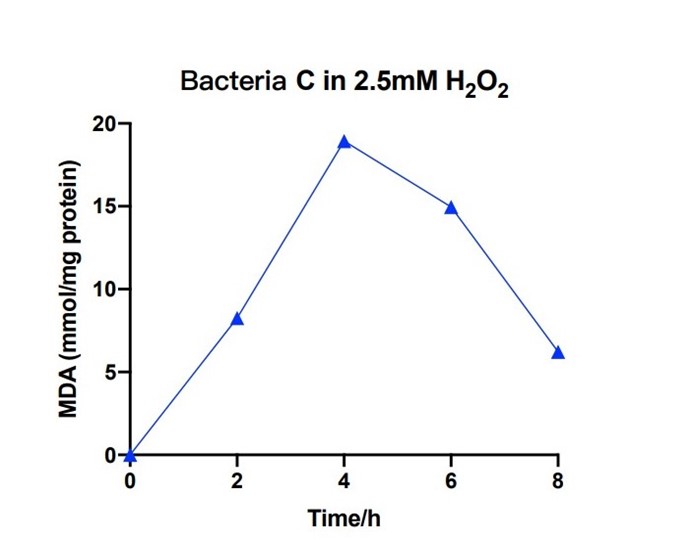
Bacteria C was placed in the 2.5nM hydrogen peroxide solution, and the level of MDA was measured under different hours. During the first 4 hours, the concentration of MDA increased up to 20 mmol/mg. The concentration of MDA decreased to 6 mmol/mg for the next four hours. The measured levels of MDA demonstrate that the efeB gene can curb the increase of MDA and keep it in a relative low level.
In order to deeper analyze the factors which may affect the ability of bacteria C and bacteria A to inhibit MDA production, more function tests would be designed and conducted in future.
Improvement of an Existing Part
Compared to the old part BBa_K592010, expressing GFP, we design a new part BBa_K3983003, which is expressing Peroxidase (efeB). The BBa_K592010 part is useful as a reporter. Peking iGEM 2016 has fused this part with triple spytag. The fused protein is participate in Peking’s polymer network. By adding this protein, the whole polymer network become visible in most conditions. The difference is that our goal is to build an engineered bacterium that can produce peroxidase efeB, which can reduce excessive reactive oxygen species produced in patients with depression by producing peroxidase, thereby reducing the possibility of depression. We chose efeB, which sequence is different from BBa_K592010 (Fig8), to construct our desired engineering strain.
First of all, we constructed a composite part BBa_K592010, and transformed it into E.coli DH5a. We successfully expressed and purified GFP protein , and detected the enzyme activity. Furthermore, in order to optimize the function of BBa_K592010,we constructed an improved composite part BBa_K3983003, which can participate in the redox process in the organism, reduce the occurrence of oxidation process, thereby reducing the active oxygen in the body of patients with depression to a certain extent.
In addition, we select a kind of probiotics E.coli DR5α as the host. Therefore, our engineering strain was a strong potential drug that can be eaten by patients for ROS and reduce the incidence of depression in patients.
Reference
[1] McCarter T. (2008). Depression overview. American health & drug benefits, 1(3), 44–51.
[2] Chand SP, Arif H. Depression. [Updated 2020 Nov 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430847/
[3] Wang, J., Wu, X., Lai, W., Long, E., Zhang, X., Li, W., Zhu, Y., Chen, C., Zhong, X., Liu, Z., Wang, D., & Lin, H. (2017). Prevalence of depression and depressive symptoms among outpatients: a systematic review and meta-analysis. BMJ open, 7(8), e017173. https://doi.org/10.1136/bmjopen-2017-017173
[4] Bueno-Notivol, J., Gracia-García, P., Olaya, B., Lasheras, I., López-Antón, R., & Santabárbara, J. (2021, January 1). Prevalence of depression during the COVID-19 outbreak: A meta-analysis of community-based studies. International Journal of Clinical and Health Psychology. DOI: 10.1016/j.ijchp.2020.07.007.
[5] U.S. Department of Health and Human Services (HHS). (n.d.). Major Depression. National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/major-depression#part_155720.
[6] Ferguson, J. M. (2001, February). SSRI Antidepressant Medications: Adverse Effects and Tolerability. Primary care companion to the Journal of clinical psychiatry. Doi: 10.4088/pcc.v03n0105
[7] Jiménez-Fernández, S., Gurpegui, M., Dí¬az-Atienza, F., Pérez-Costillas, L., Gerstenberg, M., & Correll, C. U. (2021, March 1). Comparison of ODD vs Healthy Controls. Psychiatrist.com. dx.dot.org/10.4088/JPC.14r09179.
[8] Rowe, L. A., Degtyareva, N., & Doetsch, P. W. (2008). DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free radical biology & medicine, 45(8), 1167–1177. https://doi.org/10.1016/j.freeradbiomed.2008.07.018
[9] Jiménez-Fernández, S., Gurpegui, M., Díaz-Atienza, F., Pérez-Costillas, L., Gerstenberg, M., & Correll, C. U. (2015). Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. The Journal of clinical psychiatry, 76(12), 1658–1667. https://doi.org/10.4088/JCP.14r09179
[10] Li, J., Yang, Z., Qiu, H., Wang, Y., Jian, L., Ji, J., & Li, K. (2020). Anxiety and depression among general population in China at the peak of the COVID-19 epidemic. World psychiatry : official journal of the World Psychiatric Association (WPA), 19(2), 249–250. https://doi.org/10.1002/wps.20758
[11] Vaváková, M., Ďuračková, Z., & Trebatická, J. (2015, May 20). Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2015/898393
[12] Wang Y;Li H;Li T;He H;Du X;Zhang X;Kong J; (n.d.). Cytoprotective effect of Streptococcus thermophilus against oxidative stress mediated by a novel peroxidase (EfeB). Journal of dairy science. DOI: 10.3168/jds.2018-14601
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 565
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 565
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 52
Illegal BamHI site found at 284 - 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 565
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 565
Illegal AgeI site found at 994 - 1000COMPATIBLE WITH RFC[1000]