Difference between revisions of "Part:BBa K3863004"
Alisaleong (Talk | contribs) (→Validation of Bioplastic Production) |
Alisaleong (Talk | contribs) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
This is a composite part designed to produce bioplastic PHA with his-tag. | This is a composite part designed to produce bioplastic PHA with his-tag. | ||
PHA synthase 1 from Pseudomonas resinovorans. The protein sequence in this part is based on the K2042001 sequence Evry 2016. | PHA synthase 1 from Pseudomonas resinovorans. The protein sequence in this part is based on the K2042001 sequence Evry 2016. | ||
| − | PCT is propionate CoA transferase from Clostridum propionicum. The protein sequence in this part is based on the K1211001 sequence Yale 2013. Tackling the issues with biobricks, we have optimized the codon. | + | PCT is propionate CoA transferase from <i>Clostridum propionicum</i>. The protein sequence in this part is based on the K1211001 sequence Yale 2013. Tackling the issues with biobricks, we have optimized the codon. |
| Line 14: | Line 14: | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K3863004 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3863004 SequenceAndFeatures</partinfo> | ||
| + | <html> | ||
| + | <h2>Team PuiChing_Macau 2021: Bioplastic Production</h2> | ||
| + | <h2>Validation of construct expression of K3863004</h2> | ||
| − | = | + | <img style="width:50%; height:auto" src="https://2021.igem.org/wiki/images/9/92/T--PuiChing_Macau--007PhaC.png"> |
| − | + | ||
| − | https://2021.igem.org/wiki/images/9/92/T--PuiChing_Macau--007PhaC.png | + | |
<p>Fig.1a PhaC (BBa_K3863004) qPCR</p> | <p>Fig.1a PhaC (BBa_K3863004) qPCR</p> | ||
| − | https://2021.igem.org/wiki/images/f/fe/T--PuiChing_Macau--007oPCT.png | + | |
| − | <p>Fig.1b PCT(optimized) (BBa_K3863004) qPCR</p> | + | <img style="width:50%; height:auto" src="https://2021.igem.org/wiki/images/f/fe/T--PuiChing_Macau--007oPCT.png"> |
| + | <p>Fig.1b PCT(optimized) (BBa_K3863004) qPCR </p> | ||
| + | |||
<p>Fig 1. Real-time qPCR fold change of reference gene</p> | <p>Fig 1. Real-time qPCR fold change of reference gene</p> | ||
<p>To future confirm that our constructs have the expression level, we have performed RT-qPCR | <p>To future confirm that our constructs have the expression level, we have performed RT-qPCR | ||
The RT-qPCR results show that we have successfully assembled target genes K3863004 to the vector (pETDuet).</p> | The RT-qPCR results show that we have successfully assembled target genes K3863004 to the vector (pETDuet).</p> | ||
| − | + | <h2>Validation of Bioplastic Production</h2> | |
| − | + | ||
| − | + | ||
| − | + | ||
<p>In order to observe the conversion performance, infrared spectroscopic analysis was applied since PHA and PLA have their particular functional group in chemical structure, and are performed in particular wavelengths. The absorption peak of PLA is at 1081, 1188, 1364, 1452 , 1751 cm<sup>-1[1]</sup>, whereas the absorption peak 979, 1057, 1100, 1282, 1723, 2934, 2977cm<sup>-1[2]</sup>. We have compared and analyse the peak of the wavelength to confirm the bioplastic product we have produced. | <p>In order to observe the conversion performance, infrared spectroscopic analysis was applied since PHA and PLA have their particular functional group in chemical structure, and are performed in particular wavelengths. The absorption peak of PLA is at 1081, 1188, 1364, 1452 , 1751 cm<sup>-1[1]</sup>, whereas the absorption peak 979, 1057, 1100, 1282, 1723, 2934, 2977cm<sup>-1[2]</sup>. We have compared and analyse the peak of the wavelength to confirm the bioplastic product we have produced. | ||
| Line 35: | Line 35: | ||
</p> | </p> | ||
| − | https://2021.igem.org/wiki/images/7/75/T--PuiChing_Macau--result13.jpg | + | <img style="width:100%; height:auto" src="https://2021.igem.org/wiki/images/7/75/T--PuiChing_Macau--result13.jpg"> |
| − | <p>Figure 2a. IR spectrum of pETDuet vector</p> | + | <center><p>Figure 2a. IR spectrum of pETDuet vector</p></center> |
| − | + | ||
| − | + | ||
| + | <img style="width:100%; height:auto" src="https://2021.igem.org/wiki/images/e/ed/T--PuiChing_Macau--result14.jpg"> | ||
| + | <center><p>Figure 2b. IR spectrum of BBa_K3863004</p></center> | ||
| + | <h2>Quantitative Measure of Bioplastic Production</h2> | ||
| + | <p>In order to measure the amount of bioplastic produced, we used 20 microgram Nile Red (sigma aldrich N3013) per milliliter of agar to make a Nile agar plate and streak the cell culture (BBa_K3863007(PHA), BBa_K3863004(PCT) , BBa_K3863008(Phasin)) on the plate. After incubating for 2 days at 37 celsius in darkness. Stereomicroscope (Nikon SMZ18) is used to measure the intensity of Nile red. </p> | ||
| + | <img style="width:100%; height:auto" src="https://2021.igem.org/wiki/images/c/c5/T--PuiChing_Macau--result0000.jpg"> | ||
| + | <center><p>Fig 3.Mean intensity of Nile red for quantitative measure of bioplastic production of (BBa_K3863007(PHA), BBa_K3863004(PhaC), BBa_K3863008(Phasin))</p></center> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <h2>Reference</h2> | |
<p>[1]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3450024/pdf/12088_2009_Article_31.pdf<br> | <p>[1]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3450024/pdf/12088_2009_Article_31.pdf<br> | ||
[2]https://www.researchgate.net/figure/Fourier-transform-infrared-FTIR-spectra-of-PLA-PEG-PLA-PEG-blend-and-PLA-PEG-xGnP_fig1_277675367</p> | [2]https://www.researchgate.net/figure/Fourier-transform-infrared-FTIR-spectra-of-PLA-PEG-PLA-PEG-blend-and-PLA-PEG-xGnP_fig1_277675367</p> | ||
| + | </html> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K3863004 parameters</partinfo> | <partinfo>BBa_K3863004 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
Latest revision as of 15:48, 21 October 2021
Phac-flag-PCT(optimized)-HA-his
This is a composite part designed to produce bioplastic PHA with his-tag. PHA synthase 1 from Pseudomonas resinovorans. The protein sequence in this part is based on the K2042001 sequence Evry 2016. PCT is propionate CoA transferase from Clostridum propionicum. The protein sequence in this part is based on the K1211001 sequence Yale 2013. Tackling the issues with biobricks, we have optimized the codon.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Unknown
- 21INCOMPATIBLE WITH RFC[21]Unknown
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1060
Illegal NgoMIV site found at 2125 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 251
Team PuiChing_Macau 2021: Bioplastic Production
Validation of construct expression of K3863004

Fig.1a PhaC (BBa_K3863004) qPCR

Fig.1b PCT(optimized) (BBa_K3863004) qPCR
Fig 1. Real-time qPCR fold change of reference gene
To future confirm that our constructs have the expression level, we have performed RT-qPCR The RT-qPCR results show that we have successfully assembled target genes K3863004 to the vector (pETDuet).
Validation of Bioplastic Production
In order to observe the conversion performance, infrared spectroscopic analysis was applied since PHA and PLA have their particular functional group in chemical structure, and are performed in particular wavelengths. The absorption peak of PLA is at 1081, 1188, 1364, 1452 , 1751 cm-1[1], whereas the absorption peak 979, 1057, 1100, 1282, 1723, 2934, 2977cm-1[2]. We have compared and analyse the peak of the wavelength to confirm the bioplastic product we have produced. In Figure 2, no absorption peak of PHA and PLA was identified, which indicated that the vector (pETDuet Vector) cannot give the desired product. On the other hand, the absorption peaks of PHA and PLA were identified after incubation, which suggested that the BBa_K3863004 and BBa_K3863008, the bioplastic performs significantly in the transformation process. Furthermore, the absorption peaks of PHA were found, which showed that BBa_K3863007 performs well and was the product that we expected to have. These results have proven that the bioplastic that we have produced can be formed significantly with BBa_K3863004, BBa_K3863008 and BBa_K3863007.

Figure 2a. IR spectrum of pETDuet vector

Figure 2b. IR spectrum of BBa_K3863004
Quantitative Measure of Bioplastic Production
In order to measure the amount of bioplastic produced, we used 20 microgram Nile Red (sigma aldrich N3013) per milliliter of agar to make a Nile agar plate and streak the cell culture (BBa_K3863007(PHA), BBa_K3863004(PCT) , BBa_K3863008(Phasin)) on the plate. After incubating for 2 days at 37 celsius in darkness. Stereomicroscope (Nikon SMZ18) is used to measure the intensity of Nile red.
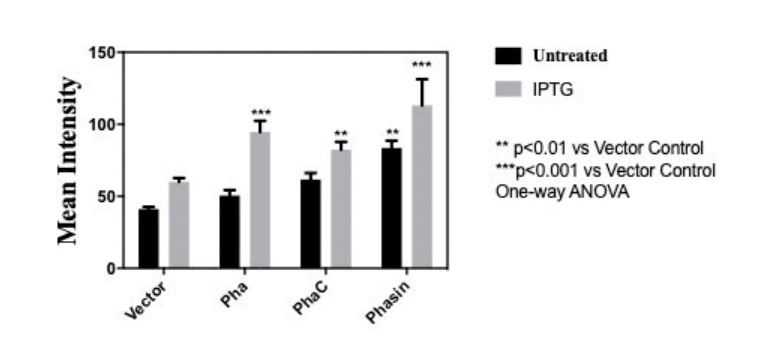
Fig 3.Mean intensity of Nile red for quantitative measure of bioplastic production of (BBa_K3863007(PHA), BBa_K3863004(PhaC), BBa_K3863008(Phasin))
Reference
[1]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3450024/pdf/12088_2009_Article_31.pdf
[2]https://www.researchgate.net/figure/Fourier-transform-infrared-FTIR-spectra-of-PLA-PEG-PLA-PEG-blend-and-PLA-PEG-xGnP_fig1_277675367
