Difference between revisions of "Part:BBa K3853050"
Lp-tiffany (Talk | contribs) (→Usage and Biology) |
|||
| (7 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K3853050 short</partinfo> | <partinfo>BBa_K3853050 short</partinfo> | ||
| + | <p>This is the coding sequence of superfolder GFP (Pedelacq et al (2006): "Engineering and characterization of a superfolder green fluorescent protein", Nature Biotech 24 (1) January 2006). This part of the amino acid sequence is the same as <partinfo>BBa_I746916</partinfo>. Using the <b>Thermo GeneArt</b> online tool geneoptimize, we optimize the codon of the protein gene sequence to improve its expression in <i>Pichia pastoris</i>. We use <partinfo>BBa_K3853055</partinfo> to construct the expression system to express and purify the protein. This can verify the ability of the AOX1 promoter.</p> | ||
| + | ===Usage and Biology=== | ||
| + | The <strong>AOX1 promoter</strong>(P<sub>AOX1</sub>) region from <em>Pichia pastoris</em>. It was first registered in 2007 and used as a strong promoter in <em>Pichia pastoris</em>. A complex pathway for the metabolism of methanol exists within some species of the <em>Komagataella</em> genus. Alcohol oxidase (AO) appears to be the first and major enzyme produced in this metabolic pathway. Transcribed from its gene (AOX1), AO converts methanol to formaldehyde within the yeast's peroxisome. | ||
| + | <p>In order to test the function of P<sub>AOX1</sub>, we construct "<strong>P<sub>AOX1</sub>-α-factor-sfGFP-AOX1 terminator"(<partinfo>BBa_K3853050</partinfo>)(Fig. 1)</strong>. If P<sub>AOX1</sub> is functional, we can test the fluorescence intensity of sfGFP in supernatant samples obtained from the culture of recombinant <em>P.pastoris</em> GS115 strain.</p> | ||
| + | <html> | ||
| + | <figure style="display: flex; justify-content: center; align-items: center;"> | ||
| + | <img src="https://2021.igem.org/wiki/images/d/d7/T--CPU_CHINA--PART_Fig_1.png" width="80%" style="float:center;"> | ||
| + | <figcaption> | ||
| + | <p style="font-size:1rem"> | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | <p style="text-align:center"><b>Fig. 1 Gene circuit of sfGFP.</b></p> | ||
| − | + | ===Characterization=== | |
| + | <p><b>1. Fluorescence intensity</b></p> | ||
| + | <p>Our results matched the general expected trend (<b>Fig 2</b>). After fermentation experiment in BMMY medium containing 0.5% methanol. The fluorescence intensity of the supernatant samples of recombinant <em>P.pastoris</em> GS115 containing the sfGFP gene gradually increased over time, while that of wild-type <em>P.pastoris</em> GS115 remained basically unchanged, which is in line with literature description<sup>[1]</sup>.</p> | ||
| + | <html> | ||
| + | <figure style="display: flex; justify-content: center; align-items: center;"> | ||
| + | <img src="https://2021.igem.org/wiki/images/7/72/T--CPU_CHINA--Parts--Contributions--fig1.png" width="70%" style="float:center;"> | ||
| + | <figcaption> | ||
| + | <p style="font-size:1rem"> | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | <p style="text-align:center"><b>Fig. 2 Fluorescence intensity of supernatant samples obtained at different time points from the culture of wild-type <em>P.pastoris</em> GS115 and corresponding recombinant <em>P.pastoris</em> GS115 containing sfGFP gene.</b></p> | ||
| + | <p><b>2. SDS-PAGE</b></p> | ||
| + | <p>SDS-PAGE results (<b>Fig. 3</b>) also verified this phenomenon, almost no protein band before 36 h could be seen. The corresponding protein band began to appear at 36 h, and the clarity and width of the protein band gradually increased over time, which means the AOX1 promoter can continuously induce the expression of the protein.</p> | ||
| + | <html> | ||
| + | <figure style="display: flex; justify-content: center; align-items: center;"> | ||
| + | <img src="https://2021.igem.org/wiki/images/f/f2/T--CPU_CHINA--Parts--Contributions--fig2.png" width="70%" style="float:center;"> | ||
| + | <figcaption> | ||
| + | <p style="font-size:1rem"> | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | <p style="text-align:center"><b>Fig. 3 SDS-PAGE gel analysis of supernatant samples of the recombinant <em>P.pastoris</em> GS115 containing the sfGFP gene during the fermentation.</b></p> | ||
| + | <P><b>3. Growth curve</b></p> | ||
| + | <p>At the same time, by measuring the growth curve of the strains (<b>Fig 4</b>), we observed that the OD<sub>600</sub> of the recombinant <em>P.pastoris</em> GS115 containing the sfGFP gene was slightly lower than the wild-type <em>P.pastoris</em> GS115 , the phenomenon of which may be attributed to the expression of sfGFP. The results showed that the expression of foreign genes would partly inhibit cell growth, but not in anintensive manner.</p> | ||
| + | <html> | ||
| + | <figure style="display: flex; justify-content: center; align-items: center;"> | ||
| + | <img src="https://2021.igem.org/wiki/images/4/44/T--CPU_CHINA--Parts--Contributions--fig3.png" width="70%" style="float:center;"> | ||
| + | <figcaption> | ||
| + | <p style="font-size:1rem"> | ||
| + | </p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | <p style="text-align:center"><b>Fig. 4 OD<sub>600</sub> absorbance obtained at different time points from the culture of wild-type <em>P.pastoris</em> GS115 and recombinant <em>P.pastoris</em> GS115 that contains sfGFP gene.</b></p> | ||
| − | + | ===References=== | |
| − | === | + | <p>[1] Xuan, Y. <em>et al.</em> An upstream activation sequence controls the expression of AOX1 gene in Pichia pastoris. <em>FEMS yeast research</em> <strong>9</strong>, 1271-1282, doi:10.1111/j.1567-1364.2009.00571.x (2009).</p> |
<!-- --> | <!-- --> | ||
Latest revision as of 14:29, 21 October 2021
PAOX1-α-factor-sfGFP-AOX1 Terminator
This is the coding sequence of superfolder GFP (Pedelacq et al (2006): "Engineering and characterization of a superfolder green fluorescent protein", Nature Biotech 24 (1) January 2006). This part of the amino acid sequence is the same as BBa_I746916. Using the Thermo GeneArt online tool geneoptimize, we optimize the codon of the protein gene sequence to improve its expression in Pichia pastoris. We use BBa_K3853055 to construct the expression system to express and purify the protein. This can verify the ability of the AOX1 promoter.
Usage and Biology
The AOX1 promoter(PAOX1) region from Pichia pastoris. It was first registered in 2007 and used as a strong promoter in Pichia pastoris. A complex pathway for the metabolism of methanol exists within some species of the Komagataella genus. Alcohol oxidase (AO) appears to be the first and major enzyme produced in this metabolic pathway. Transcribed from its gene (AOX1), AO converts methanol to formaldehyde within the yeast's peroxisome.
In order to test the function of PAOX1, we construct "PAOX1-α-factor-sfGFP-AOX1 terminator"(BBa_K3853050)(Fig. 1). If PAOX1 is functional, we can test the fluorescence intensity of sfGFP in supernatant samples obtained from the culture of recombinant P.pastoris GS115 strain.
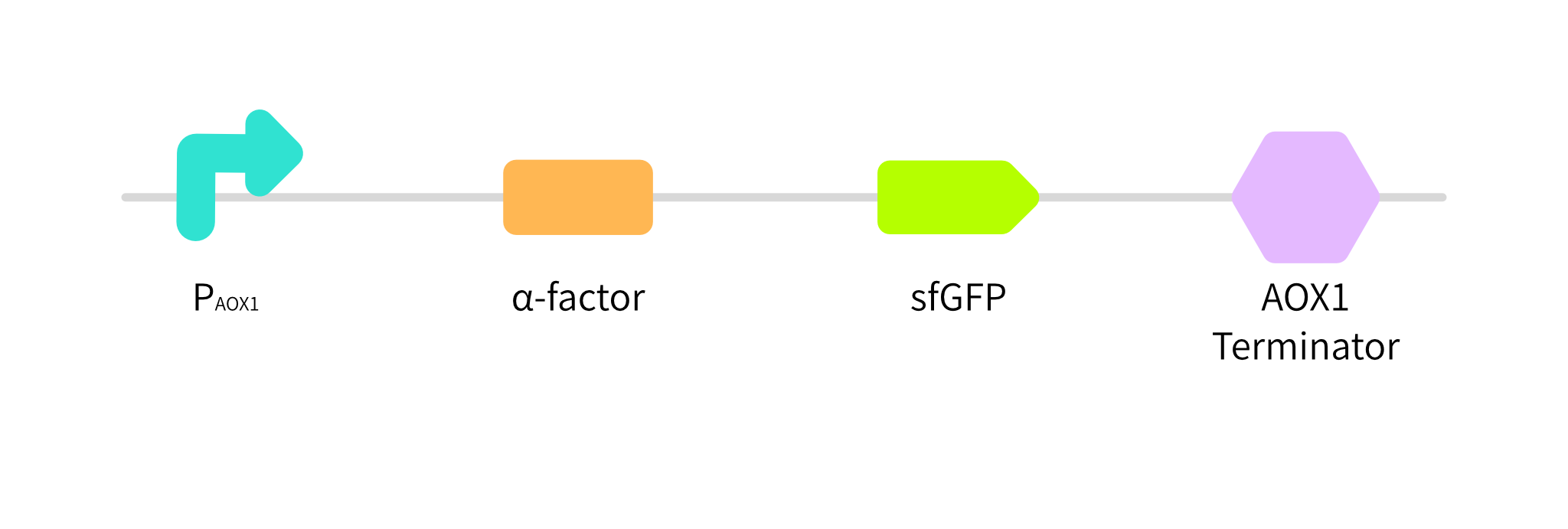
Fig. 1 Gene circuit of sfGFP.
Characterization
1. Fluorescence intensity
Our results matched the general expected trend (Fig 2). After fermentation experiment in BMMY medium containing 0.5% methanol. The fluorescence intensity of the supernatant samples of recombinant P.pastoris GS115 containing the sfGFP gene gradually increased over time, while that of wild-type P.pastoris GS115 remained basically unchanged, which is in line with literature description[1].
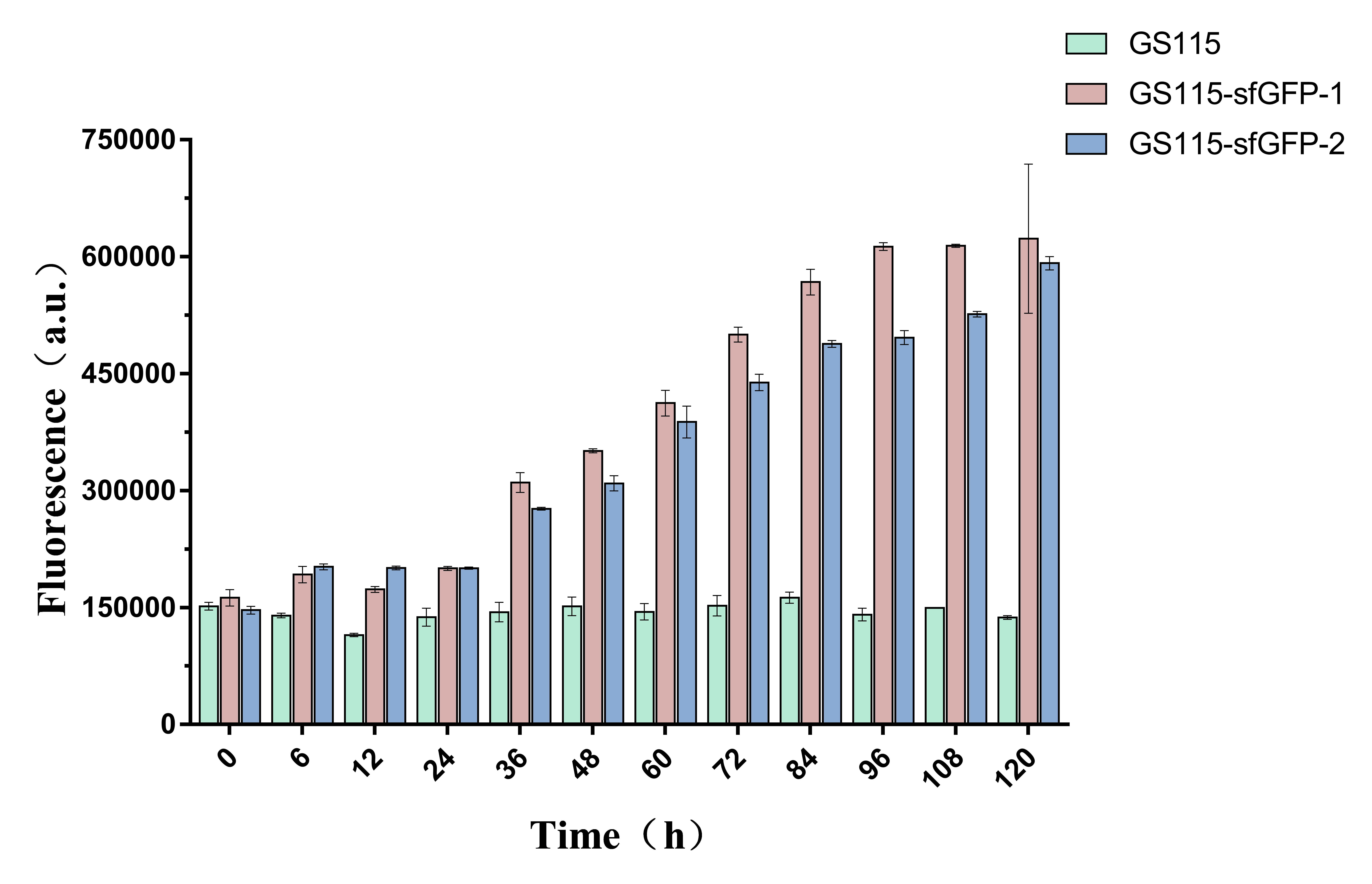
Fig. 2 Fluorescence intensity of supernatant samples obtained at different time points from the culture of wild-type P.pastoris GS115 and corresponding recombinant P.pastoris GS115 containing sfGFP gene.
2. SDS-PAGE
SDS-PAGE results (Fig. 3) also verified this phenomenon, almost no protein band before 36 h could be seen. The corresponding protein band began to appear at 36 h, and the clarity and width of the protein band gradually increased over time, which means the AOX1 promoter can continuously induce the expression of the protein.
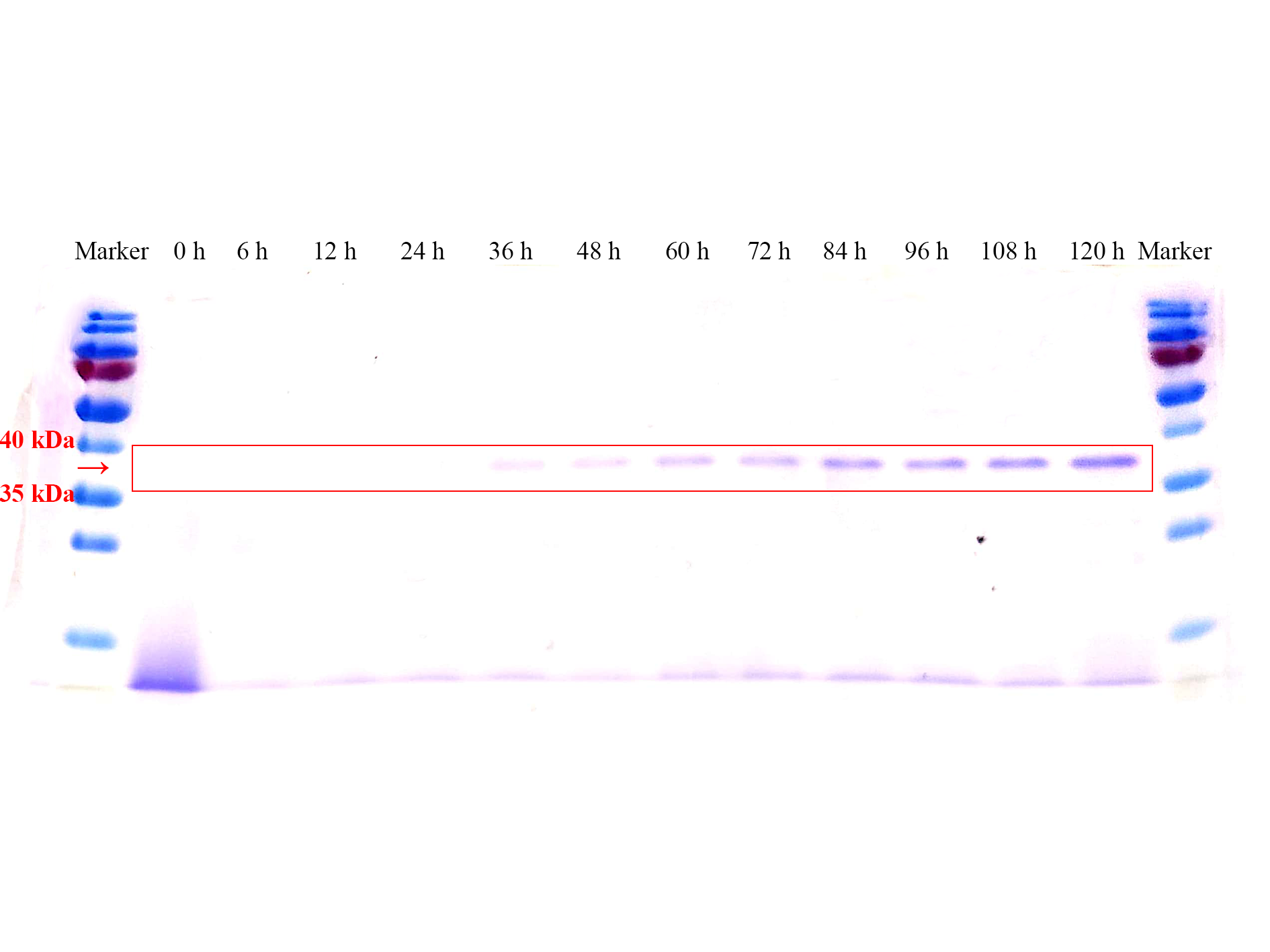
Fig. 3 SDS-PAGE gel analysis of supernatant samples of the recombinant P.pastoris GS115 containing the sfGFP gene during the fermentation.
3. Growth curve
At the same time, by measuring the growth curve of the strains (Fig 4), we observed that the OD600 of the recombinant P.pastoris GS115 containing the sfGFP gene was slightly lower than the wild-type P.pastoris GS115 , the phenomenon of which may be attributed to the expression of sfGFP. The results showed that the expression of foreign genes would partly inhibit cell growth, but not in anintensive manner.
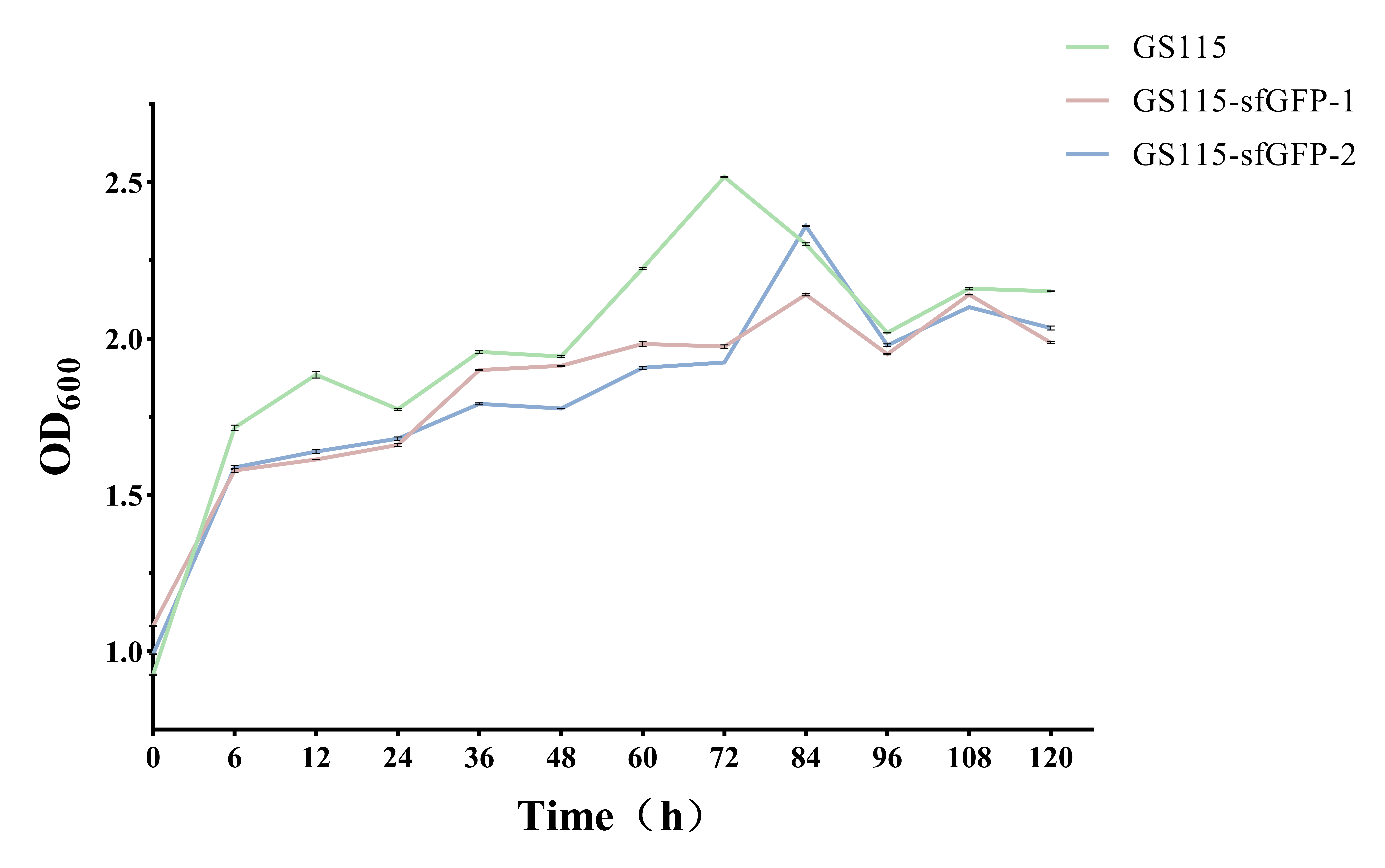
Fig. 4 OD600 absorbance obtained at different time points from the culture of wild-type P.pastoris GS115 and recombinant P.pastoris GS115 that contains sfGFP gene.
References
[1] Xuan, Y. et al. An upstream activation sequence controls the expression of AOX1 gene in Pichia pastoris. FEMS yeast research 9, 1271-1282, doi:10.1111/j.1567-1364.2009.00571.x (2009).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 1111
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
