Difference between revisions of "Part:BBa K3822005"
(→1.Construction of the plasmids) |
(→2. The effect of pEGFP-miR-196a-148a 1.0 and pEGFP-miR-196a-148a 2.0 in gastric cancer cells) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 14: | Line 14: | ||
==1.Construction of the plasmids== | ==1.Construction of the plasmids== | ||
The miRNA “ sponge” method was introduced by several years ago as a means to create continuous miRNA loss of function in cell lines and transgenic organisms. More effective are sponges containing bulged sites that are mispaired opposite miRNA positions 9-12 (Ebert et al. 2007, Gentner et al. 2009), presumably because they form a more stable interaction with the miRNA. pEGFP-miR196a-148a 2.0(Part K3822005) was designed based on the principle of effective sponges containing bulged sites (Fig. 1). We compare pEGFP-miR196a-148a 2.0 with pEGFP-miR196a-148a 1.0 (Part K3160007,designed by a 2019 iGEM team) on monitoring the expression of miRNAs in gastric cancer cells. | The miRNA “ sponge” method was introduced by several years ago as a means to create continuous miRNA loss of function in cell lines and transgenic organisms. More effective are sponges containing bulged sites that are mispaired opposite miRNA positions 9-12 (Ebert et al. 2007, Gentner et al. 2009), presumably because they form a more stable interaction with the miRNA. pEGFP-miR196a-148a 2.0(Part K3822005) was designed based on the principle of effective sponges containing bulged sites (Fig. 1). We compare pEGFP-miR196a-148a 2.0 with pEGFP-miR196a-148a 1.0 (Part K3160007,designed by a 2019 iGEM team) on monitoring the expression of miRNAs in gastric cancer cells. | ||
| − | [[File:T--YiYe-China--Improve1.png|400px|thumb|center| Fig 1. Pairing of a miRNA with a bulged sponge site shows mismatches opposite miRNA nucleotides.]] | + | [[File:T--YiYe-China--Improve1.png|400px|thumb|center| <b>Fig 1. Pairing of a miRNA with a bulged sponge site shows mismatches opposite miRNA nucleotides.</b>]] |
| + | The plasmids of pEGFP-miR-196a-148a 2.0 was synthesized by Nanjing Qingke Biotechnology Corporation. The electrophoresis of the plasmids was showed in Fig 2. | ||
| + | [[File:T--YiYe-China--Improve2.png|400px|thumb|center| <b>Fig 2. Electrophoresis of pEGFP-miR-196a-148a 2.0.</b>]] | ||
| + | ==2. The effect of pEGFP-miR-196a-148a 1.0 and pEGFP-miR-196a-148a 2.0 in gastric cancer cells== | ||
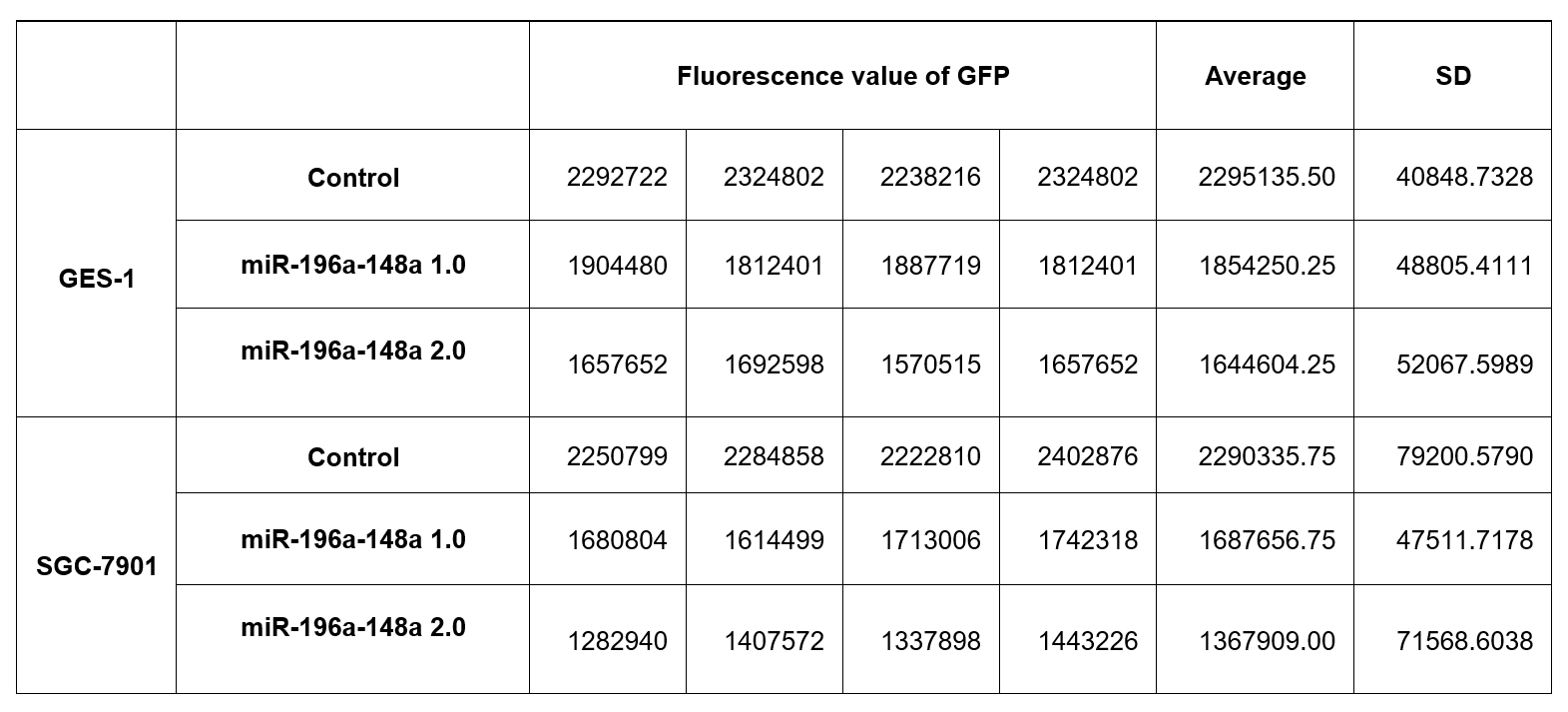
| + | To detect the validity of pEGFP-miR-196a-148a 1.0 and pEGFP-miR196a-148a 2.0 in cells, pEGFP–C1 (as negative controls), pEGFP-miR-196a-148a 1.0 or pEGFP-miR-196a-148a 2.0 (0.5 ug plasmids for each well) was transfected into human gastric epithelial cells (GES-1 cells) in 24-well plate, respectively. After transfection, cells were examined under fluorescence microscopy (Fig 3A-C). The fluorescence of GFP was decreased in GES-1 cells transfected with pEGFP-miR-196a-148a 1.0 and pEGFP-miR-196a-148a 2.0 compared with controls (Fig 3 A-C). Because pEGFP-miR-196a-148a 2.0 contains more effective binding sites than pEGFP-miR-196a-148a 1.0, the fluorescence of GFP was further decreased in GES-1 cells transfected with pEGFP-miR-196a-148a 2.0 compared with that in cells transfected with pEGFP-miR-196a-148a 1.0 (Fig. 3 and Table 1). In addition, we also measured the value of GFP fluorescence by plate reader (SpectraMax i3) (Fig. 3-4 and Table. 1). The similar results were also observed in SGC-7901 cells (gastric cancer cell line) (Fig. 3-4, and Table 1). Compared with pEGFP-miR-196a-148a 1.0, pEGFP-miR-196a-148a 2.0 is more effective to be inhibited by the endogenous miRNAs (Fig. 3 and Table. 1). | ||
| + | [[File:T--YiYe-China--Improve3.png|600px|thumb|center|<b> Fig 3. The images of different gastric cells transfected with different plasmids. </b>(A). pEGFP–C1 (control) was transfected in GES-1 cells. (B). pEGFP-miR-196a-148a 1.0 was transfected in GES-1 cells. (C). pEGFP-miR-196a-148a 2.0 was transfected in GES-1 cells. (D). pEGFP–C1 (control) was transfected in SGC-7901 cells. (E). pEGFP-miR-196a-148a sensor 1.0 was transfected in SGC-7901 cells. (F). pEGFP-miR-196a-148a sensor 2.0 was transfected in SGC-7901 cells.]] | ||
| + | <p style="font-size: 14px; text-align: center; color: black;"> <b>Table 1. The value of GFP fluorescence in cells.</b> | ||
| + | [[File:T--YiYe-China--Improvetable1.png|600px|thumb|center]] | ||
| + | [[File:T--YiYe-China--Improve4.png|600px|thumb|center|<b> Fig 4. The value of GFP fluorescence in cells. </b>Cells were transfected with pEGFP–C1, pEGFP-miR-196a-148a 1.0 or pEGFP-miR-196a-148a 2.0 for 24 h. GFP fluorescence was measured by SpectraMax i3.]] | ||
| + | In order to estimate the effect of pEGFP-miR-196a-148a sensor 1.0 and pEGFP-miR-196a-148a sensor 2.0 on reflecting the different expression of miR-196a and miR-148a in gastric cancer cells compared with normal cells, we reanalyzed the data of table1. The down-regulation of GFP fluorescence was observed in gastric cancer cells transfected with miRNA sensors compared with normal cells (Fig 5). Taken together, these results reveal a possibility of detecting tumor cells by using pEGFP-miR-196a-148a sensor 2.0. | ||
| + | |||
| + | [[File:T--YiYe-China--Improve5.png|600px|thumb|center|<b> Fig 5. The value of GFP fluorescence in different cells transfected with pEGFP –C1, pEGFP-miR-196a-148a 1.0 or pEGFP-miR-196a-148a 2.0.</b>]] | ||
| + | ==3. The effect of miR-196a-148a 2.0 as a monitor to detect the expression of miR-148a== | ||
| + | To test the effect of miR-196a-148a 2.0 as a monitor to detect the expression of miR-148a, cells were transfected with pEGFP-miR-196a-148a 1.0 or pEGFP-miR-196a-148a 2.0 with different concentration of pHAGE-pre-miR-148a (overexpression of miR-148a). We used the plasmid of pHAGE-pre-miR-148a to quantify miR-148a expression and calculate the copy numbers of miR-148a by using the formula listed below. | ||
| + | |||
| + | copies/ul= (6.02×1023)×(plasmids concentrations ng/ul×10-9)/(DNA length×660) | ||
| + | |||
| + | [[File:T--YiYe-China--Improvetable2.png|600px|thumb|center|<b>Table 2. The value of eGFP fluorescence of pEGFP-miR-196a-148a 1.0.</b>]] | ||
| + | |||
| + | [[File:T--YiYe-China--Improvetable3.png|600px|thumb|center|<b>Table 3. The value of eGFP fluorescence of pEGFP-miR-196a-148a 2.0</b>]] | ||
| + | |||
| + | The standard curve of miR-196a-148a sensor 1.0 was made by EXCEL (Fig. 6). We find that the value of fluorescence is dependent on the copy numbers of miR-148a in cells. Based on the formula, the correlation coefficient (R2 value) of miR-196a-148a sensor was 0.9462, the slope of it was -29851. | ||
| + | |||
| + | [[File:T--YiYe-China--Improve6.png|600px|thumb|center|<b> Fig 6. The standard curve of miR-196a-148a 1.0.</b>]] | ||
| + | |||
| + | The standard curve of miR-196a-148a 2.0 was also made by EXCEL (Fig. 7). We find that the value of fluorescence is also dependent on the copy numbers of miR-148a in cells. Based on the formula, the correlation coefficient (R2 value) of miR-196a-148a sensor was 0.9578, the slope was -37508. By comparing the slope, we found that miR-196a-148a sensor 2.0 is more suitable for the detection of the expression of miR-148a. | ||
| + | [[File:T--YiYe-China--Improve7.png|600px|thumb|center|<b> Fig 7. The standard curve of miR-196a-148a 2.0.</b>]] | ||
| + | |||
| + | ==Reference:== | ||
| + | [1] Ebert et al. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells.Nat Methods. 2007 Sep;4(9):721-6.doi: 10.1038/nmeth1079. Epub 2007 Aug 12. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
<partinfo>BBa_K3822005 parameters</partinfo> | <partinfo>BBa_K3822005 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
Latest revision as of 02:48, 15 October 2021
miR196a-148a 2.0
miR196a-148a 2.0 is an improvement of Part BBa_K3160007. which could monitor the expression of miRNAs in gastric cancer cells
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Result
1.Construction of the plasmids
The miRNA “ sponge” method was introduced by several years ago as a means to create continuous miRNA loss of function in cell lines and transgenic organisms. More effective are sponges containing bulged sites that are mispaired opposite miRNA positions 9-12 (Ebert et al. 2007, Gentner et al. 2009), presumably because they form a more stable interaction with the miRNA. pEGFP-miR196a-148a 2.0(Part K3822005) was designed based on the principle of effective sponges containing bulged sites (Fig. 1). We compare pEGFP-miR196a-148a 2.0 with pEGFP-miR196a-148a 1.0 (Part K3160007,designed by a 2019 iGEM team) on monitoring the expression of miRNAs in gastric cancer cells.
The plasmids of pEGFP-miR-196a-148a 2.0 was synthesized by Nanjing Qingke Biotechnology Corporation. The electrophoresis of the plasmids was showed in Fig 2.
2. The effect of pEGFP-miR-196a-148a 1.0 and pEGFP-miR-196a-148a 2.0 in gastric cancer cells
To detect the validity of pEGFP-miR-196a-148a 1.0 and pEGFP-miR196a-148a 2.0 in cells, pEGFP–C1 (as negative controls), pEGFP-miR-196a-148a 1.0 or pEGFP-miR-196a-148a 2.0 (0.5 ug plasmids for each well) was transfected into human gastric epithelial cells (GES-1 cells) in 24-well plate, respectively. After transfection, cells were examined under fluorescence microscopy (Fig 3A-C). The fluorescence of GFP was decreased in GES-1 cells transfected with pEGFP-miR-196a-148a 1.0 and pEGFP-miR-196a-148a 2.0 compared with controls (Fig 3 A-C). Because pEGFP-miR-196a-148a 2.0 contains more effective binding sites than pEGFP-miR-196a-148a 1.0, the fluorescence of GFP was further decreased in GES-1 cells transfected with pEGFP-miR-196a-148a 2.0 compared with that in cells transfected with pEGFP-miR-196a-148a 1.0 (Fig. 3 and Table 1). In addition, we also measured the value of GFP fluorescence by plate reader (SpectraMax i3) (Fig. 3-4 and Table. 1). The similar results were also observed in SGC-7901 cells (gastric cancer cell line) (Fig. 3-4, and Table 1). Compared with pEGFP-miR-196a-148a 1.0, pEGFP-miR-196a-148a 2.0 is more effective to be inhibited by the endogenous miRNAs (Fig. 3 and Table. 1).

Table 1. The value of GFP fluorescence in cells.
In order to estimate the effect of pEGFP-miR-196a-148a sensor 1.0 and pEGFP-miR-196a-148a sensor 2.0 on reflecting the different expression of miR-196a and miR-148a in gastric cancer cells compared with normal cells, we reanalyzed the data of table1. The down-regulation of GFP fluorescence was observed in gastric cancer cells transfected with miRNA sensors compared with normal cells (Fig 5). Taken together, these results reveal a possibility of detecting tumor cells by using pEGFP-miR-196a-148a sensor 2.0.
3. The effect of miR-196a-148a 2.0 as a monitor to detect the expression of miR-148a
To test the effect of miR-196a-148a 2.0 as a monitor to detect the expression of miR-148a, cells were transfected with pEGFP-miR-196a-148a 1.0 or pEGFP-miR-196a-148a 2.0 with different concentration of pHAGE-pre-miR-148a (overexpression of miR-148a). We used the plasmid of pHAGE-pre-miR-148a to quantify miR-148a expression and calculate the copy numbers of miR-148a by using the formula listed below.
copies/ul= (6.02×1023)×(plasmids concentrations ng/ul×10-9)/(DNA length×660)
The standard curve of miR-196a-148a sensor 1.0 was made by EXCEL (Fig. 6). We find that the value of fluorescence is dependent on the copy numbers of miR-148a in cells. Based on the formula, the correlation coefficient (R2 value) of miR-196a-148a sensor was 0.9462, the slope of it was -29851.
The standard curve of miR-196a-148a 2.0 was also made by EXCEL (Fig. 7). We find that the value of fluorescence is also dependent on the copy numbers of miR-148a in cells. Based on the formula, the correlation coefficient (R2 value) of miR-196a-148a sensor was 0.9578, the slope was -37508. By comparing the slope, we found that miR-196a-148a sensor 2.0 is more suitable for the detection of the expression of miR-148a.
Reference:
[1] Ebert et al. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells.Nat Methods. 2007 Sep;4(9):721-6.doi: 10.1038/nmeth1079. Epub 2007 Aug 12.