Difference between revisions of "Part:BBa K3332060"
Huangzinuo (Talk | contribs) |
AnnaTaylor (Talk | contribs) |
||
| Line 69: | Line 69: | ||
<html> | <html> | ||
<figure> | <figure> | ||
| − | <img src="https://2020.igem.org/wiki/images/4/ | + | <img src="https://2020.igem.org/wiki/images/4/40/T--XMU-China--XMU-China_2020-GRHPR-BrkA.png |
| + | |||
| + | g"width="40%" style="float:center"> | ||
<figcaption> | <figcaption> | ||
<p style="font-size:1rem"> | <p style="font-size:1rem"> | ||
Revision as of 23:41, 27 October 2020
J23100-RBS-GRHPR-BrkA-terminator
We anchor GRHPR protein onto membranes through BrkA to catalyze the reaction of reducing glyoxalic acid and consuming NADPH.
Biology
BrkA is an anchor protein from Bordetella pertussis, which has β-barrel structure. It can anchor its passenger protein to the cell membrane and has been widely used in cell-surface display. GRHPR, a glyoxylate reductase from human liver, can reduce glyoxylic acid when NADPH is used as cofactor.
GRHPR is fused at C terminal with BrkA so that GRHPR can be displayed on the surface of E. coli.[1][2]
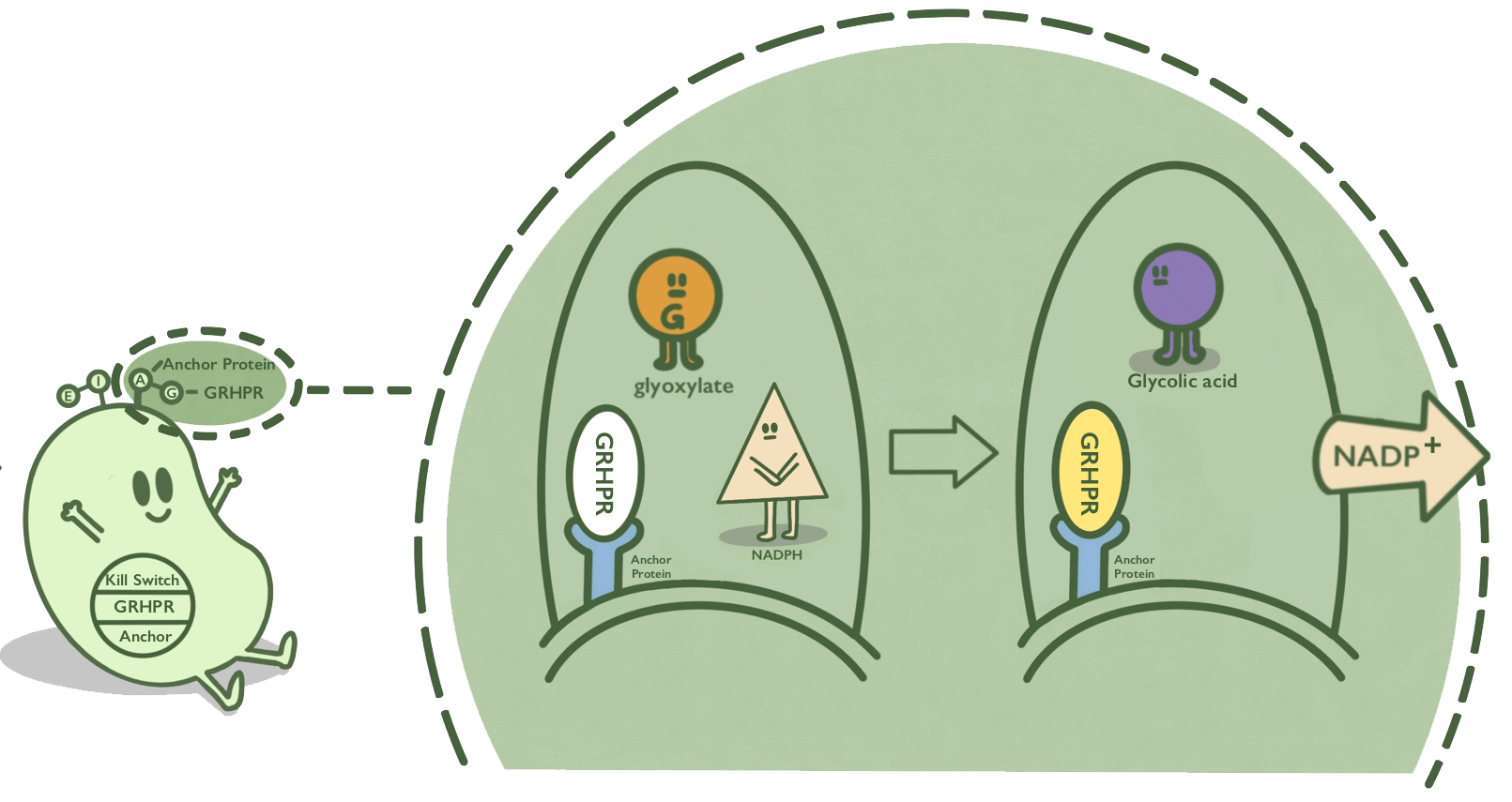
- Fig 1. Mechanism of GRHPR on the surface of E. coli.
Usage
Here, we used K880005 to construct the expression system and demonstrated the effect of GRHPR-BrkA on the surface of E. coli. We obtained the composite part BBa_K3332060 and transformed the constructed plasmid into E. coli BL21 (DE3) to verify its expression. The positive clones were cultivated.

- Fig 2. Gene circuit of GRHPR-BrkA.
Characterization
1.Identification
After receiving the synthesized DNA, restriction digestion was done to certify that the plasmid was correct, and the experimental results were shown in figure 3.

- Fig 3. DNA gel electrophoresis of restriction digest products of GRHPR-BrkA-pSB1C3 (Xbal I & Pst I sites).
2. The proof of expression
We used J23100 promoter to highly express GRHPR-BrkA in E. coli in our composite part BBa_K3332060. In order to verify whether our recombinant plasmid works, we use membrane protein extraction kit to get membrane protein.
Then, our target bands are observed through SDS-PAGE and the experimental results are shown in figure 4. Notably, in GRHPR-BrkA lane we could see our target protein band which don’t show up in negative controls.

- Fig 4. SDS-PAGE of membrane protein extraction products of GRHPR-BrkA-pSB1C3.
3. Ability of consuming NADPH
We mixed glyoxylic acid solution, NADPH solution and bacteria solution carrying GRHPR-BrkA. Then, we immediately measured OD340 changes. TECAN® Infinite M200 Pro was used to detect OD340. And when NADPH is consumed, OD340 declines.
We successfully got OD340-Time curves of GRHPR fused with 4 types of anchor protein. When using GRHPR-BrkA, we could see OD340 decrease as the reaction went on. However, by using J23100-RBS and GRHPR-Histag as control, we could also find that the slopes of these three curves are similar. The results show that our fusion protein GRHPR-BrkA do not work well. The result is shown in figure 5.

- Fig 5. OD340-Time curves of GRHPR fused with 4 types of anchor protein.
References
- ↑ Rumsby G, Cregeen D P. Identification and expression of a cDNA for human hydroxypyruvate/glyoxylate reductase[J]. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression, 1999, 1446(3): 383-388.
- ↑ http://2016.igem.org/Team:TJUSLS_China
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1358
Illegal NgoMIV site found at 1787
Illegal NgoMIV site found at 2423
Illegal AgeI site found at 608 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 2391
