Difference between revisions of "Part:BBa K3595004"
Liyingying (Talk | contribs) |
|||
| (7 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
The wild-type cysE gene encodes L-serine O-acetyl transferase (SAT), which is a key enzyme in the pathway of hydrogen sulfide metabolism.In the pathway of converting hydrogen sulfide to L-cysteine, L-serine and Acetyl-CoA form O-acetylserine catalyzed by SAT. | The wild-type cysE gene encodes L-serine O-acetyl transferase (SAT), which is a key enzyme in the pathway of hydrogen sulfide metabolism.In the pathway of converting hydrogen sulfide to L-cysteine, L-serine and Acetyl-CoA form O-acetylserine catalyzed by SAT. | ||
| − | <!-- Add more about the biology of this part here | + | <!-- Add more about the biology of this part here--> |
| − | + | =Usage and Biology= | |
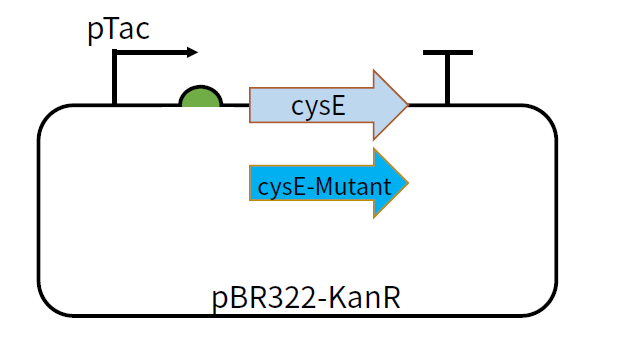
| + | This part is coding sequence after the promoter pTac and RBS B0034. The feedback inhibition-insensitive SAT can be translated under the induction of IPTG. We constructed plasmids pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-Mutant,among which the mutants include cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2. The constructed plasmid was transformed into <i>Nissle </i> host cell to test its production of cysteine. | ||
| + | [[File:T--GZ_HFI--cysE.png|600px|thumb|center|The structure of the plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant ]] | ||
| + | ==Experimental Setup== | ||
| + | *Genetic information of cysE,cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2 was described on the page of [[Part:BBa_K3595005]],[[Part:BBa_K3595006]],[[Part:BBa_K3595007]], [[Part:BBa_K3595008]],[[Part:BBa_K3595009]], [[Part:BBa_K3595010]], [[Part:BBa_K3595011]],respectively.[[Part:BBa_K731000]] | ||
| + | *Plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant was transfered into the <i>Nissle </i> host cell,respestively. | ||
| + | *Single colonies were selected from the experimental LB-agar plate , then inoculated into test-tube tubes with 4000 μL LB liquid medium with 4uL kanamycin for overnight growth at 37 °C and 200 rpm. | ||
| + | *Inoculating 15 uL of culture solution overnight into a 24-well plate containing 3 mL M9 medium for overnight growth at 37 °C and 200 rpm.The media contained 3 ul kanamycin and 1.5 uL 1M IPTG. At the same time, wild-type Nissle was inoculated as negative control, and M9 medium was used as blank control. | ||
| + | *Detecting cysteine concentration in culture medium | ||
| + | ==Results== | ||
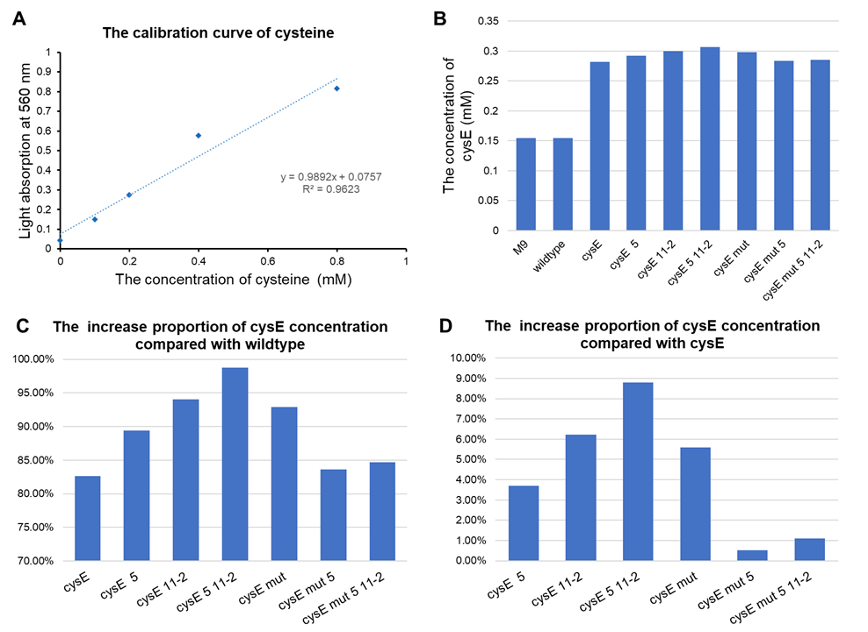
| + | *All of our engineered bacteria have shown great improvement in the ability to produce cysteine, which represents that they can absorb H2S to a larger extent. | ||
| + | *EcN with plasmid pTYT-cysE-5-11-2 had the best effect, 98.72% batter than EcN wildtype and 8.79% better than overexpression of cysE (pTYT-cysE) | ||
| + | [[File:T--GZ_HFI--H2S.png|600px|thumb|center|Detection results of the effect of the hydrogen sulfide pathway. (A) The calibration line of cysteine. (B) Concentration of cysteine detected in each group. (C) The ratio of increased cysteine concentration in each group compared to wildtype. (D) Cysteine production compared to pTYT-cysE. CysE-mut in the Figure stands for cysE-256. ]] | ||
<!-- --> | <!-- --> | ||
| Line 12: | Line 24: | ||
<partinfo>BBa_K3595004 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3595004 SequenceAndFeatures</partinfo> | ||
| + | <!-- Add more about the biology of this part here--> | ||
| + | =Reference= | ||
| + | Kondoh M, et al. L-Cysteine production by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 2019;103(6):2609-19. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 21:34, 27 October 2020
cysE-Wildtype
The wild-type cysE gene encodes L-serine O-acetyl transferase (SAT), which is a key enzyme in the pathway of hydrogen sulfide metabolism.In the pathway of converting hydrogen sulfide to L-cysteine, L-serine and Acetyl-CoA form O-acetylserine catalyzed by SAT.
Usage and Biology
This part is coding sequence after the promoter pTac and RBS B0034. The feedback inhibition-insensitive SAT can be translated under the induction of IPTG. We constructed plasmids pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-Mutant,among which the mutants include cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2. The constructed plasmid was transformed into Nissle host cell to test its production of cysteine.
Experimental Setup
- Genetic information of cysE,cysE-256, cysE-5, cysE-11-2, cysE-5-11-2, cysE-256-5,cysE-256-11-2,cysE-256-5-11-2 was described on the page of Part:BBa_K3595005,Part:BBa_K3595006,Part:BBa_K3595007, Part:BBa_K3595008,Part:BBa_K3595009, Part:BBa_K3595010, Part:BBa_K3595011,respectively.Part:BBa_K731000
- Plasmid pBR322-KanR-pTac-cysE and pBR322-KanR-pTac-cysE-mutant was transfered into the Nissle host cell,respestively.
- Single colonies were selected from the experimental LB-agar plate , then inoculated into test-tube tubes with 4000 μL LB liquid medium with 4uL kanamycin for overnight growth at 37 °C and 200 rpm.
- Inoculating 15 uL of culture solution overnight into a 24-well plate containing 3 mL M9 medium for overnight growth at 37 °C and 200 rpm.The media contained 3 ul kanamycin and 1.5 uL 1M IPTG. At the same time, wild-type Nissle was inoculated as negative control, and M9 medium was used as blank control.
- Detecting cysteine concentration in culture medium
Results
- All of our engineered bacteria have shown great improvement in the ability to produce cysteine, which represents that they can absorb H2S to a larger extent.
- EcN with plasmid pTYT-cysE-5-11-2 had the best effect, 98.72% batter than EcN wildtype and 8.79% better than overexpression of cysE (pTYT-cysE)
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 684
- 1000COMPATIBLE WITH RFC[1000]
Reference
Kondoh M, et al. L-Cysteine production by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 2019;103(6):2609-19.