Difference between revisions of "Part:BBa K3521000"
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[SHSID-part.png:SHSID-part.png|200px|thumb|left|alt text]] | [[SHSID-part.png:SHSID-part.png|200px|thumb|left|alt text]] | ||
| + | __NOTOC__ | ||
| + | <partinfo>BBa_K3521000 short</partinfo> | ||
| + | |||
| + | <partinfo>BBa_K3521000 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | ===Contribution=== | ||
| + | ===Recombinant FnCpf1 production, purification, and SDS-PAGE analysis=== | ||
| + | |||
| + | The backbone for BBa_K3521005 was derived from pET-28a vector. The recombinant plasmid was transformed into BL21 (DE3) competent cells and induced with IPTG. After induction, a specific protein band that is consistent with the theoretical molecular weight of FnCpf1 was detected in SDS-PAGE (Figure 2). The recombinant FnCpf1 was successfully purified by Ni-affinity chromatography. | ||
| + | [[File:T--SHSID-BBa_K3521005 Fig 0.jpg|600px|thumb|left|Figure 1. pET28a-Cas12a(FnCpf1)]] | ||
| + | |||
| + | [[File:T--SHSID-BBa_K3521005 Fig 1.jpg|600px|thumb|left|Figure 2. SDS-PAGE analysis of FnCpf1 production in BL21 (DE3) cells. After induction, cells were collected by centrifugation and broken by sonication. The resulting supernatants were loaded to the PAGE gel with different amounts. Lane2 1-4: the samples from the cultures induced with 1 mM IPTG; Lane2 5-8: the samples from the cultures induced with 0.5 IPTG; lane 9: protein molecular weight standards.]] | ||
Latest revision as of 19:11, 27 October 2020
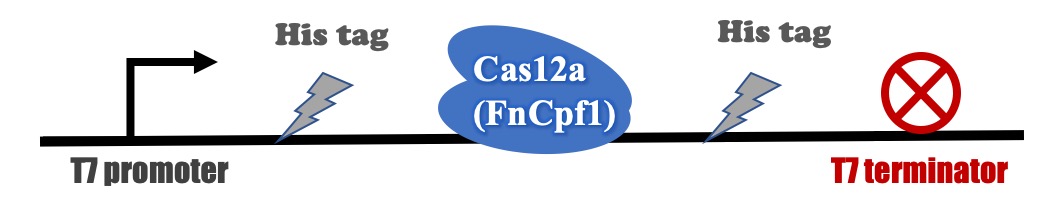
T7 promoter
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Contribution
Recombinant FnCpf1 production, purification, and SDS-PAGE analysis
The backbone for BBa_K3521005 was derived from pET-28a vector. The recombinant plasmid was transformed into BL21 (DE3) competent cells and induced with IPTG. After induction, a specific protein band that is consistent with the theoretical molecular weight of FnCpf1 was detected in SDS-PAGE (Figure 2). The recombinant FnCpf1 was successfully purified by Ni-affinity chromatography.

Figure 2. SDS-PAGE analysis of FnCpf1 production in BL21 (DE3) cells. After induction, cells were collected by centrifugation and broken by sonication. The resulting supernatants were loaded to the PAGE gel with different amounts. Lane2 1-4: the samples from the cultures induced with 1 mM IPTG; Lane2 5-8: the samples from the cultures induced with 0.5 IPTG; lane 9: protein molecular weight standards.