Difference between revisions of "Part:BBa K3552013"
(→Characterization) |
|||
| (5 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
SccJ is a ribozyme or insulator which can eliminate the interference mediated by the input promoter. We used three different ribozymes to measure their different ability of improving the reaction of ptac promoter. We calculate the value and compare the gradient of the graph of fluorescent against concentration of IPTG. | SccJ is a ribozyme or insulator which can eliminate the interference mediated by the input promoter. We used three different ribozymes to measure their different ability of improving the reaction of ptac promoter. We calculate the value and compare the gradient of the graph of fluorescent against concentration of IPTG. | ||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- --> | <!-- --> | ||
| Line 13: | Line 9: | ||
<partinfo>BBa_K3552013 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3552013 SequenceAndFeatures</partinfo> | ||
| + | <!-- --> | ||
| + | ===Characterization=== | ||
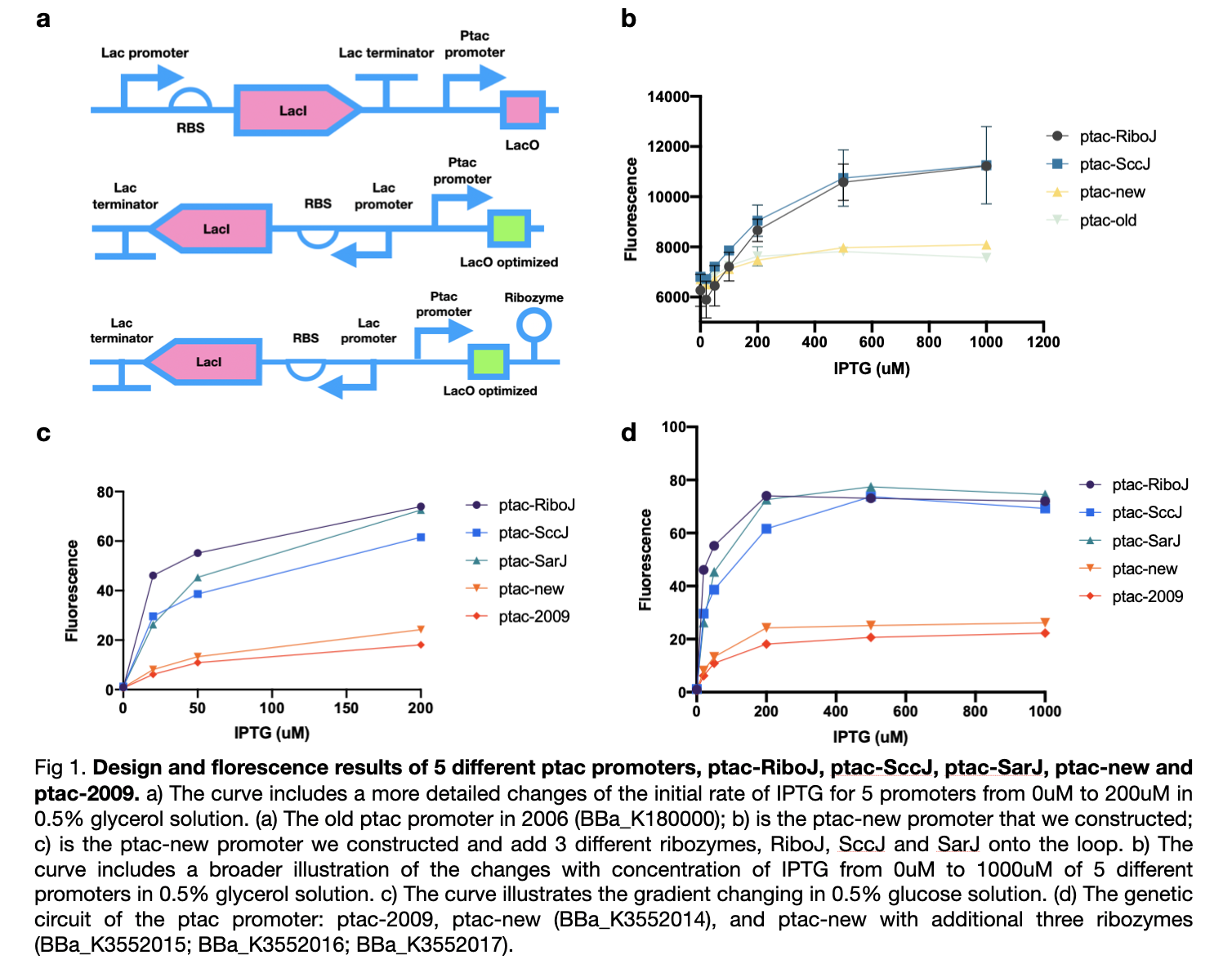
| + | This year we improved the previous ptac promoter, part <partinfo>BBa_K180000</partinfo>, found in 2009 by reversing its lacI promoter, RBS, and terminator which will improve its yield of production. We also added three types of ribozymes, RiboJ, SccJ, and SarJ as an insulator to prevent the previous effect from the ptac promoter. | ||
| + | |||
| + | We first carried out an experiment in the culture medium with glucose as the source of carbon for the cultivation of bacteria in the M9 medium. In this experiment, we tested four ptac promoter, the old one, the new one, the one with RiboJ, and with SccJ. We convinced that all for ptac promoters are able to be activated when induced by IPTG, and as the concentration of IPTG increases, the expression of GFP genes promotes. Furthermore, the addition of two different types of ribozyme shows a prominent improvement of sensitivity towards the concentration of IPTG. At zero concentration of IPTG, all four promoter starts at a similar level, as the concentration of IPTG increases, the gradient of the graph increased dramatically. | ||
| + | |||
| + | Afterward, according to our own medium, which utilizes glycerol as the only source of carbon, we attempted to simulate this situation for all five types of ptac promoter to investigate whether they can fit our experiments or not. Therefore, we substituted the medium to glycerol-M9 medium. The collected results imply that all five promoters can be activated obviously and the same as in glucose, promoters with ribozyme also obtain high sensitivity to the concentration of IPTG and dramatically increased. | ||
| + | |||
| + | [[File:T--LINKS_China--Figure 5 different ptac promoter.jpeg|600px]] | ||
| + | |||
| + | These results confirmed the effect of our sensors under various types of carbon sources and maintain nearly no difference in practicability. We modified four new ptac promoter parts which are convinced to have higher sensitivity and adapt better to IPTG than the <partinfo>BBa_K180000</partinfo> discovered in 2009. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 13:45, 27 October 2020
SccJ
SccJ is a ribozyme or insulator which can eliminate the interference mediated by the input promoter. We used three different ribozymes to measure their different ability of improving the reaction of ptac promoter. We calculate the value and compare the gradient of the graph of fluorescent against concentration of IPTG.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
This year we improved the previous ptac promoter, part BBa_K180000, found in 2009 by reversing its lacI promoter, RBS, and terminator which will improve its yield of production. We also added three types of ribozymes, RiboJ, SccJ, and SarJ as an insulator to prevent the previous effect from the ptac promoter.
We first carried out an experiment in the culture medium with glucose as the source of carbon for the cultivation of bacteria in the M9 medium. In this experiment, we tested four ptac promoter, the old one, the new one, the one with RiboJ, and with SccJ. We convinced that all for ptac promoters are able to be activated when induced by IPTG, and as the concentration of IPTG increases, the expression of GFP genes promotes. Furthermore, the addition of two different types of ribozyme shows a prominent improvement of sensitivity towards the concentration of IPTG. At zero concentration of IPTG, all four promoter starts at a similar level, as the concentration of IPTG increases, the gradient of the graph increased dramatically.
Afterward, according to our own medium, which utilizes glycerol as the only source of carbon, we attempted to simulate this situation for all five types of ptac promoter to investigate whether they can fit our experiments or not. Therefore, we substituted the medium to glycerol-M9 medium. The collected results imply that all five promoters can be activated obviously and the same as in glucose, promoters with ribozyme also obtain high sensitivity to the concentration of IPTG and dramatically increased.
These results confirmed the effect of our sensors under various types of carbon sources and maintain nearly no difference in practicability. We modified four new ptac promoter parts which are convinced to have higher sensitivity and adapt better to IPTG than the BBa_K180000 discovered in 2009.