Difference between revisions of "Part:BBa K3349007"
Kristiturton (Talk | contribs) |
Kristiturton (Talk | contribs) |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3349007 short</partinfo> | <partinfo>BBa_K3349007 short</partinfo> | ||
| − | This Pectin Lyase is a | + | This Pectin Lyase is a protein from <i> paenibacillus amylolyticus</i>. With Pnl and PelC, PelB is able to cleave and therefore breakdown pectin or homogalacturonan substrates. This protein has been characterized to cleave methylated pectin (at unmethylated sites) and polygalacturonic acid [2]. Studies by Keggi and Doran-Peterson, PelB has a Km of 50.1+/- 140 mg/ml and a Vmax of 745 +/-2024 IU/mg with a 80% methylated substrate. The optimal temperature for PelB activity was determined to be 70°C [1]. |
| + | |||
| + | A hexa-histidine tag was added onto the C-terminus for nickel affinity purification. For more information, please visit our website https://2020.igem.org/Team:Lethbridge_HS. | ||
| + | |||
| + | Although this enzyme is already fairly thermostable we decided to do homology modelling if we were to apply our modelling protocol to make a new PelB thermostable variant. | ||
| − | |||
<html> | <html> | ||
| Line 12: | Line 15: | ||
</html> | </html> | ||
| − | <b>Figure | + | <b>Figure 1:</b> Homology modelling of PelB from <i>P. amylolyticus</i>. The protein structure was done using iTASSER [2] and SWISS-Model [3]. The two structures were then aligned and compared. A more in depth analysis can be found on our webpage. |
| + | |||
| + | <html> | ||
| + | <img src= "https://2020.igem.org/wiki/images/c/c9/T--Lethbridge_HS--insertdigest2020.png" alt="pnl modelling" style="width:500px;height:700px;"> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | <b>Figure 2: </b> Restriction digest of PelB and PelC DNA from the pUC57 plasmid using EcoRI and PstI enzymes run on a 1% agarose gel. The red boxes show the band corresponding to that of our DNA constructs. | ||
| + | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 16:50, 24 October 2020
Pectin Lyase PelB
This Pectin Lyase is a protein from paenibacillus amylolyticus. With Pnl and PelC, PelB is able to cleave and therefore breakdown pectin or homogalacturonan substrates. This protein has been characterized to cleave methylated pectin (at unmethylated sites) and polygalacturonic acid [2]. Studies by Keggi and Doran-Peterson, PelB has a Km of 50.1+/- 140 mg/ml and a Vmax of 745 +/-2024 IU/mg with a 80% methylated substrate. The optimal temperature for PelB activity was determined to be 70°C [1].
A hexa-histidine tag was added onto the C-terminus for nickel affinity purification. For more information, please visit our website https://2020.igem.org/Team:Lethbridge_HS.
Although this enzyme is already fairly thermostable we decided to do homology modelling if we were to apply our modelling protocol to make a new PelB thermostable variant.
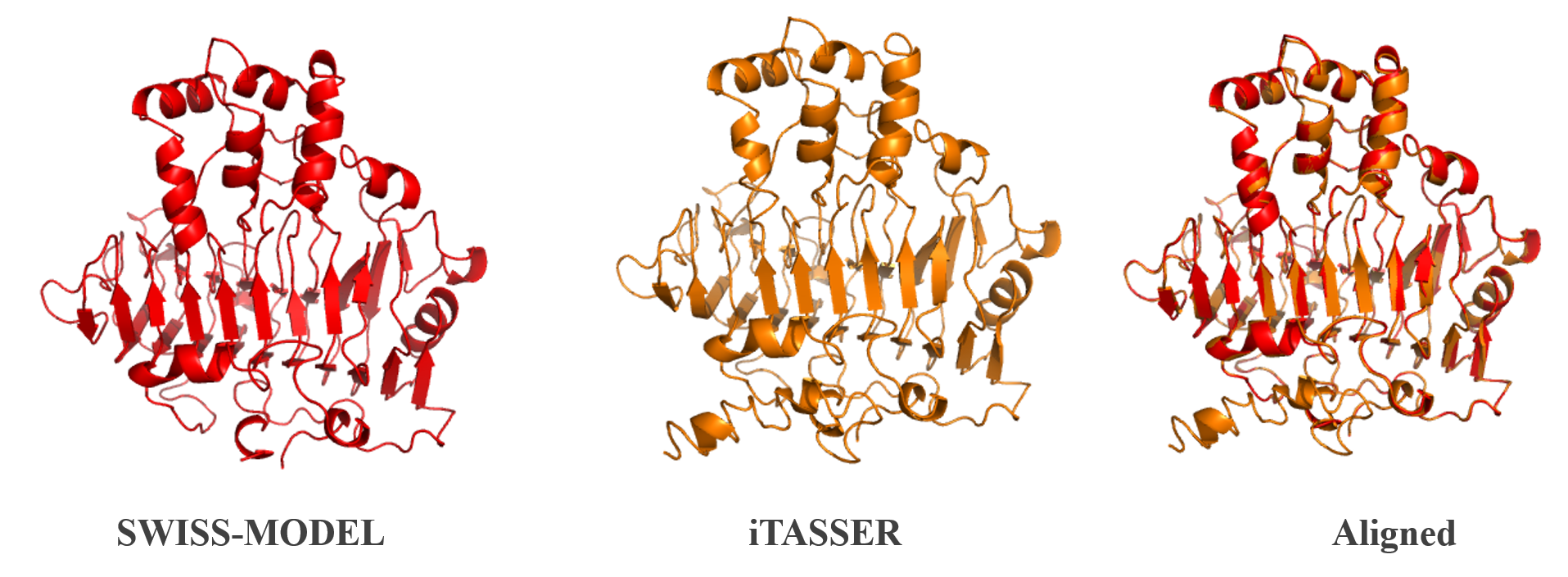
Figure 1: Homology modelling of PelB from P. amylolyticus. The protein structure was done using iTASSER [2] and SWISS-Model [3]. The two structures were then aligned and compared. A more in depth analysis can be found on our webpage.
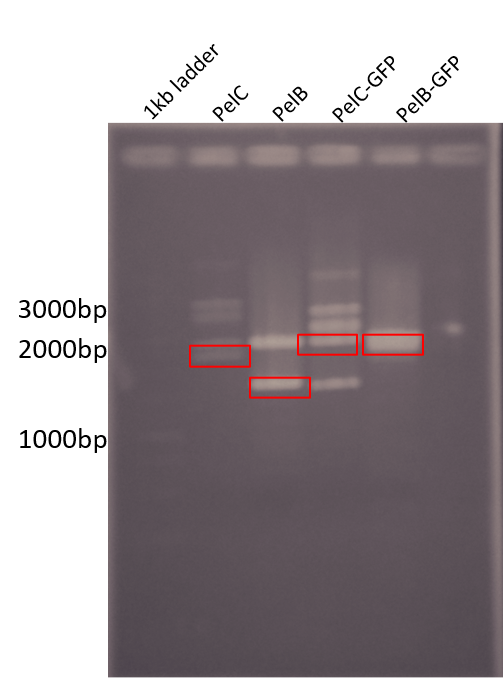
Figure 2: Restriction digest of PelB and PelC DNA from the pUC57 plasmid using EcoRI and PstI enzymes run on a 1% agarose gel. The red boxes show the band corresponding to that of our DNA constructs.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 333
Illegal BamHI site found at 1218 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
