Difference between revisions of "Part:BBa J01003:Experience"
(→Characterization) |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
This experience page is provided so that any user may enter their experience using this part.<BR>Please enter | This experience page is provided so that any user may enter their experience using this part.<BR>Please enter | ||
how you used this part and how it worked out. | how you used this part and how it worked out. | ||
| − | + | ==Applications of BBa_J01003== | |
| + | |||
| + | ==Characterization of BBa_J01003== | ||
| + | |||
| + | We have characterized the oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] in a conjugation system in which pUB307 is used as helper plasmid and ''E. coli'' as donor and recipient cells. We measured quantitatively the conjugative competence of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] by conjugation between different strains, at different temperatures and with different donor/recipient ratios. We also compared the conjugation kinetics of [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] and pUB307 quantitatively. | ||
| + | |||
| + | The part oriT R [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] was cotransformed together with pUB307 (helper plasmid) into ''E. coli'' strain Top10. These cells therefore became conjugation donor and have a kanamycin (from pUB307) and ampicillin (from [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003]) resistance. The recipient cells have the plasmid pBAD33 with a chloramphenicol resistance. After mixing both cell types the conjugation starts and [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] as well as pUB307 can be transported. The helper plasmid pUB307 can still be transported since it still contains its oriT region. The transconjugants which have received [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] through the conjugation have ampicillin and chloramphenicol resistance and can therefore be easily selected on agar plates containing these two antibiotics. | ||
| + | |||
| + | |||
| + | ===Test 1 Conjugation kinetics of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] between ''E. coli'' Top10=== | ||
| + | |||
| + | On the first day we inoculated LB + antibiotics medium with a single colony or from a glycerol stock. For this test we used donor and the recipient cells that were both ''E. coli'' Top10. On the second day we washed the cells of the overnight culture twice with LB medium, transferred the donor cell suspension to the pellet of the recipient cells and resuspended the recipient cells in this medium. After another centrifugation step we resuspended the pellet which now is a mixture of donor and recipient cells in 100 µl LB medium without antibiotics. We than transferred the cell suspension on a membrane filter, which was placed on LB plates before and incubated these plates at 37 °C for 0, 6, 12, 18, 24, 30, 36, 42 minutes. Afterwards the membrane was put into 1 ml LB medium and the cells were resuspended by vortexing for 30 seconds. The cell suspension was diluted 10<sup>-5</sup> and plated on the LB / ampicillin + chloramphenicol (CA) plates. The plates were incubated at 37 °C overnight and on the third day the colonies could be counted. <br> | ||
| + | We have done this test thrice independently and calculated the conjugation efficient which is defined as [transconjugants per ml] / [donor per ml] from this assay. <br> | ||
| + | To count the donor and recipient cell density small aliquots of the used suspensions were diluted 10<sup>-7</sup> and plated on agar plates containing kanamycin + ampicillin for donor cells and chloramphenicol for the recipients. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Table 1:''' Conjugation kinetics of oriT BBa_J01003 between ''E. coli'' Top10. | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=0px. width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Group|| colspan="2" align="center"|Test A|| colspan="2" align="center"|Test B || colspan="2" align="center"| Test C | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell|| Donor ||Recipient|| Donor || Recipient || Donor || Recipient | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Strain|| Top10|| Top10|| Top10 || Top10 || Top10 || Top10 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell number|| 9325 || 8100|| 3465 || 8330 || 7140 || 7470 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Time [min]|| Transconjugants ||Efficiency|| Transconjugants || Efficiency || Transconjugants || Efficiency | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |0|| 150 ||0.016|| 9 || 0,003 || 37 || 0,005 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |6|| 500 || 0.054|| 20 || 0,006 || 390 || 0,055 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |12|| 780 ||0.084|| 94 || 0,027 || 1390 || 0,195 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |18|| 1160||0.124|| 172 || 0,050 || 1720 || 0,241 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |24|| 1400||0.150|| 262 || 0,076 || 2880 || 0,403 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |30|| 3360||0.360|| 1450 || 0,418 || 3500 || 0,490 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |36|||| || 1790 || 0.517 || 3750 || 0,525 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |42|| |||| 2170 || 0.626 || || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[Image:HD_kinetic of conjugation.png|center|thumb|500px|'''Figure 1: conjugation kinetic of [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] between ''E. coli'' Top10 strains.''' Note that the conjugation efficiency increases with the time allowed for the conjugation to take place.]] | ||
| + | |||
| + | Figure 1 shows that the kinetic of the conjugative plasmid [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] has three phases. In the first (0 min to 24 min) and in the third phase (30 min to 42 min) the curve has a smaller slope, whereas the second phase (24 min to 30 min) it has a very big slope. An explanation for this result could be that the cell growth in the first 24 min can be neglected due to the average generation time of ''E. coli'' in the LB medium which lies between 20 and 30 minutes whereas in the second phase the growth of ''E. coli'' dominates the conjugation process and leads to this deviation from linearity. In this test we used very concentrated bacteria suspension in a small conjugation volume which leads to the observation of conjugation in an approximately linear process in the primary phase.<br> | ||
| + | We have done a linear regression analysis for the first phase (0 min to 24 min) and we calculated the slope for this phase as 9.00 • 10<sup>-3</sup> [transconjugants • donor<sup>-1</sup> • minute <sup>-1</sup>]. We calculated the conjugation rate for [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] which is defined as transconjugants • donor<sup>-1</sup> • recipient<sup>-1</sup> • minute <sup>-1</sup>. The conjugation rate of [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] is 1.05 • 10<sup>-12</sup> [ml • min<sup>-1</sup> • cell<sup>-1</sup>]. This rate was used by the modeling group as a parameter value. <br> | ||
| + | Figure 1 also shows us that the transport of the plasmid pSB1A2 containing the part [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] by conjugation is a very fast process since we got transconjugants in the first minutes of the experiment. Through this experiment we can conclude that the transport of the plasmid pSB1A2 containing [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] by conjugation needs only about 2 minutes. | ||
| + | Since we have two conjugative plasmids in our donor cells, we wanted to see which plasmid is preferred to be transported by conjugation. We therefore plated the cells in the test C (see table 2) also on agar plates containing chloramphenicol + kanamycin (CK) and chloramphenicol + kanamycin + ampicillin (CKA) to select for transconjugants getting pUB307 and both plasmids. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Table 2:''' Test C conjugation kinetics of oriT BBa_J01003 and pUB307 between ''E. coli'' Top10. | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=0px. width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Select|| colspan="2" align="center" |Chlorophencol+Kanamycin (J01103) ||colspan="2" align="center" |Chlorophencol+Kanamycin (pUB307) || colspan="2" align="center" | Chlorophenicol+Ampicilin+Kanamycin | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell|| Donor || Recipient|| Donor || Recipient || Donor || Recipient | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Strain|| Top10|| Top10||Top10 || Top10 || Top10 || Top10 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell number|| 7140||7470|| 7140 || 7470 || 7140 || 7470 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Time [min]|| Transconjugants ||Efficiency|| Transconjugants || Efficiency || Transconjugants || Efficiency | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |0|| 37 ||0.005|| 2 || 0,000 || 1 || 0,000 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |6|| 390 || 0.055|| 17 || 0,002 || 4 || 0,001 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |12|| 1390 || 0.195|| 194 || 0,027 || 90 || 0,013 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |18|| 1720 ||0.241|| 230 || 0,032 || 109 || 0,015 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |24|| 2880 || 0.403|| 1130 || 0,158 || 800 || 0,112 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |30|| 3500|| 0.490|| 2500 || 0,350 || 1540 || 0,216 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |36|| 3750 ||0.525|| 2600 || 0,364 || 1800 || 0,252 | ||
| + | |-style="height:20px" | ||
| + | | || || || || || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[Image:HD_conjugation efficiency of different plasmids.png|center|thumb|500px|'''Figure 2: Conjugation efficiency of different plasmids.''' Transconjugants with [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] were selected by plating on LB agar containing chloramphenicol and ampicillin, transconjugants with pUB307 by plates containing chloramphenicol and kanamycin, and transconjugants with both plasmids by plates containing chloramphenicol and kannamycin and ampicillin.]] | ||
| + | |||
| + | |||
| + | Figure 2 clearly shows that the plasmid pSB1A2 containing only [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] is preferably transported during the first 20 minutes in comparison to pUB307. Since pUB307 has the same oriT as [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] is, we assume the size of the plasmid to be the reason for this since [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] in pSB1A2 is approx. 2.5 kb in size whereas pUB307 has 60 kb. A comparison of the red and the green curve shows us that the difference in the duration of the transport of plasmids with different size is not very big. Looking at the blue curve you can see that the first transconjugants with both plasmids can be detected after approx. 18 minutes which is longer than the sum of both transport times. | ||
| + | |||
| + | |||
| + | ===Test 2 Conjugation kinetics of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] between different ''E.coli'' strains=== | ||
| + | |||
| + | For this experiment we used the same technique as described in test 1. In this case we used different ''E. coli'' strains as recipients, namely ''E. coli'' Top10, MG1655 and DH5α. In this experiment the cells on the membranes were incubated for 0, 6, 12, 18, 24, 30, 36 and 42 minutes at 37 °C. | ||
| + | This test was conducted twice independently and the conjugation efficiency calculated as described in test 1. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Table 3:''' Conjugation kinetics of oriT BBa_J01004 between different ''E. coli'' strains. | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=0px. width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Group|| colspan="3" align="center" | Test D (Donor: Top10; Cell number: 5440)||colspan="3" align="center" |Test E (Donor: Top10; Cell number: 7140) | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell|| Recipient||Recipient|| Recipient || Recipient || Recipient || Recipient | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Strain||Top10 || MG1655|| DH5alpha || Top10 || MG1655 || DH5alpha | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell number|| 6086 || 5566|| 5096 || 7470 || 7275 || 6900 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Time [min]|| Efficiency||Efficiency|| Efficiency || Efficiency || Efficiency || Efficiency | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |0|| 0.002 || 0,000|| 0,000 || 0,005 || 0,000 || 0,001 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |6|| 0.002 ||0,000|| 0,000 || 0,055 || 0,000 || 0,003 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |12|| 0.003 ||0,000|| 0,000 || 0,195 || 0,001 || 0,067 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |18|| 0.005 ||0,000|| 0,001 || 0,241 || 0,007 || 0,165 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |24|| 0.021 ||0,000|| 0,018 || 0,403 || 0,019 || 0,269 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |30|| 0.071 ||0,001|| 0,075 || 0,490 || 0,036 || 0,415 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |36|| 0.136 ||0,013|| 0,246 || 0,525 || 0,129 || 0,482 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |42|| ||0.041|| 0,301 || || 0,134 || 0,566 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[Image:HD_different strains.png|center|thumb|500px|'''Figure 3: Conjugation of [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] in pSB1A2 between different strains of ''E. coli''.''' The transconjugants were selected through growing on plates containing chloramphenicol and ampicillin. Note that conjugation from Top10 to Top10 and DH5α is comparable whereas conjugation in MG1655 takes place a lot slower.]] | ||
| + | |||
| + | |||
| + | As you can see in figure 3 there is a difference in the conjugation kinetic between Top10 as donor and different strains as recipient. With ''E. coli'' Top10 as donor ''E. coli'' DH5α is a comparably good recipient as Top10 whereas MG1655 showed up as a bad recipient under these conditions. Comparing the red with the blue curve one can see that DH5α transconjugants appear later (6 minutes) that Top10, but the conjugation efficiency and also the conjugation rate (slope between 6 and 24 minutes) are in the same range with Top10 and DH5α. We assume that more time is needed for stabilizing the pillus contact between Top10 and DH5α than between two Top10 cells, but the conjugation between Top10 and DH5α can be done as good as between two Top10 cells. Comparing the red and the green curve one can see that the first MG1655 transconjugants appear much later (20 minutes) and the conjugation efficiency and rate (slope between 20 and 32 minutes) are much smaller. Therefore we conclude that conjugation between different strains is possible using this oriT but the efficiency depends on the used strains and their compatibility. | ||
| + | |||
| + | |||
| + | ===Test 3 Conjugative competence of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] at different donor/recipient ratios=== | ||
| + | |||
| + | In this experiment we again used the same protocol as described above in test 1 but used different donor / recipient rations, namely 50:1, 25:1, 10:1, 8:1, 5:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:5, 1:10, 1:25, and 1:50. This test was conducted twice independently and the conjugation efficiency calculated as described above. The donor and recipient cell density was measured by plating 10<sup>-7</sup> dilutions on agar plates containing kanamycin + ampicillin for the donor and chloramphenicol for recipient cells. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Table 4:''' Conjugative competence of otiT BBa_J01003 at different donor / recipient ratios. | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=0px. width=100px | || width=100px | || width=100px | || width=100px | || width=100px | || width=100px | || width=100px | || width=100px | || width=100px | | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Group|| colspan="4" align="center" | Test F || colspan="4" align="center" | Test G | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Anticipant ratio|| Donor || Recipient|| Actual ratio || Transconjugants || Donor || Recipient || Actual ratio || Transconjugants | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |50:1|| 1003 || 23|| 43:1 || 12 || 832 || 21 || 40:1 || 16 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |25:1|| 982 || 46|| 21.5:1 || 28 || 815 || 41 || 20:1 || 31 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |10:1|| 930 || 110|| 8.5:1 || 62 || 729 || 97 || 7.5:1 || 63 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |8:1|| 909 || 133|| 7:1 || 81 || 711 || 117 || 6:1 || 75 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |5:1|| 854 || 202|| 4:1 || 115 || 665 || 153 || 4.5:1 || 120 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |3:1|| 767||302|| 2.5:1 || 161 || 599 || 266 || 2.3:1 || 129 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |2:1|| 680||403|| 1.7:1 || 213 || 533 || 355 || 1.5:1 || 212 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:1|| 510||605|| 1:1.2 || 302 || 400 || 532 || 1:1.3 || 303 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:2|| 340||802|| 1:2.3 || 351 || 268 || 705 || 1:2.6 || 298 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:3|| 257||902|| 1:3.5 || 294 || 199 || 794 || 1:4 || 302 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:5|| 170||1003|| 1:5.9 || 289 || 133 || 883 || 1:6.7 || 194 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:8|| 122||1072|| 1:8.8 || 241 || 89 || 943 || 1:10.5 || 190 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:10|| 94||1095|| 1:11.7 || 158 || 72 || 963 || 1:13 || 134 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:25|| 40||1159|| 1:29 || 86 || 31 || 1020 || 1:33 || 82 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |1:50|| 20||1182|| 1:59 || 31 || 16 || 1040 || 1:67 || 30 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[Image:HD_different ratio.png|center|thumb|500px|'''Figure 4: Conjugative competence of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] at different donor / recipient ratios.''' For all of the samples a similar cell density was used. For the conjugation a time of 20 minutes was set. The transconjugants were selected by plating on plates containing chloramphenicol and ampicillin.]] | ||
| + | |||
| + | |||
| + | Figure 4 shows very clearly that at same cell density, if donor : recipient ratio is 1 : 2 the highest amount of transconjugants can be achieved. A big difference in the amount of donor : recipient leads to a low number of transconjugants. At low donor : recipient ratios the conjugation efficiency was quiet high but lead to a low number of transconjugants since the density of donor cells was too low. Only at donor : recipient rations between 1 : 1 and 1 : 3, a high conjugation efficiency and a high donor density was achieved leading to a high number of transconjugants. A maximum in the number of transconjugants could be achieved at a ratio of 1 : 2. | ||
| + | |||
| + | |||
| + | ===Test 4 Conjugative competence of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] at different temperatures=== | ||
| + | |||
| + | In this experiment the same method is used as described above in test 1. Here, donor and recipient both were ''E. coli'' Top10. The incubation during conjugation took place at different temperatures, namely 4 °C, 22 °C, 30 °C, 37 °C and 42 °C for 20, 40 and 60 minutes. For counting of transconjugants the cell suspension was diluted 10<sup>-6</sup> and plated on agar containing ampicillin and chloramphenicol (CA). This experiment was conducted twice independently. The donor and recipient cell density was again calculated by plating 10<sup>-7</sup> dilutions on plates containing kanamycin and ampicillin or chloramphenicol, respectively. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Table 5:''' Conjugative competence of otiT BBa_J01003 at different temperatures. | ||
| + | {| class="wikitable" | ||
| + | |- bgcolor=grey | ||
| + | ! height=0px. width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | || width=120px | | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Group|| colspan="3" align="center" | Test H|| colspan="3" align="center" |Test J | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell|| Donor || Recipient|| donor : recipient || Donor || Recipient || donor/recipient | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Strain|| Top10||Top10|| 1 : 2.02 || Top10 || Top10 || 1:1.77 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Cell number|| 361||731|| sum:1092 || 330 || 584 || sum:914 | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Sample||colspan="3" align="center" | Transconjugants || colspan="3" align="center" | Transconjugants | ||
| + | |-align="center" bgcolor=grey | ||
| + | | style="font-weight:bold;" |Temperature|| 10min||40min|| 60min || 20min || 40min || 60min | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |4°C|| 5|| 5|| 1 || 2 || 1 || 0 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |22°C|| 2||78|| 117 || 29 || 61 || 79 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |30°C|| 71 ||178|| 241 || 43 || 117 || 227 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |37°C|| 154 ||285|| 550 || 118 || 290 || 332 | ||
| + | |-align="center" | ||
| + | | style="font-weight:bold;" |42°C|| 167|| 273|| 402 || 122 || 271 || 234 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | [[Image:HD_different temperature.png|center|thumb|500px|'''Figure 5: Conjugative competence of oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] at different temperatures.''' All of the samples have the similar cell density and similar donor : recipient ratio. The conjugation time was set to 20, 40 and 60 minutes. Transconjugants are selected by plating on the agar containing chloramphenicol and ampicillin. Note, that the conjugation efficiency after 20 and 40 minutes is the same at 37 °C and 42 °C but differs after 60 minutes.]] | ||
| + | |||
| + | |||
| + | As you can see in figure 5, conjugation is most efficient at 37 °C. From 0 to 20 minutes during the primary conjugation phase the cell growth can be neglected. From 20 to 40 minutes, the cell growth is also presented in the conjugation curves. At 4 °C, the conjugation curve has a very small slope (near zero) and no significant transconjugants production could be observed. At 22, 30, 37 and 42 °C, a significant number of transconjugants could be achieved, but the conjugation efficiency and the conjugation rate (slope between 0 and 20 minutes) differ strongly. From 22 °C to 37°C, the conjugation efficiency and conjugation rate increases with rising temperature. Comparing these three curves between 20 and 40 minutes one can see that the cell growth gets more important with rising temperature. Dynamically, the cells have more mobility at higher temperature, and have a bigger possibility to touch another cell, which is necessary for conjugation. When we compared the conjugation curve at 42 °C with the one at 37 °C, one can see, that the conjugation efficient and the conjugation rate at 42 °C is as good as at 37 °C. After the primary conjugation phase, the production of transconjugants at 42 °C is clearly worse than the production at 37 °C what can be seen at comparing the curves between 40 and 60 minutes. A possible reason for this could be a lower growth and higher death rate at 42 °C compared to 37 °C. So, one can conclude, that for best conjugation efficiency one needs a warm environment for the conjugation to take place but also temperatures which favor cell growth. | ||
| + | |||
| + | |||
| + | ===Summary=== | ||
| + | |||
| + | The characterization of [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] in pSB1A2 has given the following results: | ||
| + | * The conjugation has an average conjugation rate of 1.05 • 10<sup>-12</sup> [ml • min<sup>-1</sup> • cell<sup>-1</sup>] | ||
| + | * The conjugation has an approximate transport time of 2 minutes | ||
| + | * The oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] will be transported first in attendance of pUB307 in the donor cells | ||
| + | * Smaller plasmids favor the conjugation and are conjugated first | ||
| + | * The oriT [https://parts.igem.org/wiki/index.php?title=Part:BBa_J01003 BBa_J01003] can be conjugated between Top10 cells and from Top10 to DH5α cells with similar efficiency but from Top10 to MG1655 with a strongly lower efficiency | ||
| + | * The conjugation favors a donor : recipient ratio of 1 : 2 | ||
| + | * The conjugation has the best production of transconjugants, the best efficiency and the best conjugation rate at 37 °C | ||
| − | + | ==User Reviews== | |
<!-- DON'T DELETE --><partinfo>BBa_J01003 StartReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_J01003 StartReviews</partinfo> | ||
<!-- Template for a user review | <!-- Template for a user review | ||
Latest revision as of 15:09, 29 October 2008
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J01003
Characterization of BBa_J01003
We have characterized the oriT BBa_J01003 in a conjugation system in which pUB307 is used as helper plasmid and E. coli as donor and recipient cells. We measured quantitatively the conjugative competence of oriT BBa_J01003 by conjugation between different strains, at different temperatures and with different donor/recipient ratios. We also compared the conjugation kinetics of BBa_J01003 and pUB307 quantitatively.
The part oriT R BBa_J01003 was cotransformed together with pUB307 (helper plasmid) into E. coli strain Top10. These cells therefore became conjugation donor and have a kanamycin (from pUB307) and ampicillin (from BBa_J01003) resistance. The recipient cells have the plasmid pBAD33 with a chloramphenicol resistance. After mixing both cell types the conjugation starts and BBa_J01003 as well as pUB307 can be transported. The helper plasmid pUB307 can still be transported since it still contains its oriT region. The transconjugants which have received BBa_J01003 through the conjugation have ampicillin and chloramphenicol resistance and can therefore be easily selected on agar plates containing these two antibiotics.
Test 1 Conjugation kinetics of oriT BBa_J01003 between E. coli Top10
On the first day we inoculated LB + antibiotics medium with a single colony or from a glycerol stock. For this test we used donor and the recipient cells that were both E. coli Top10. On the second day we washed the cells of the overnight culture twice with LB medium, transferred the donor cell suspension to the pellet of the recipient cells and resuspended the recipient cells in this medium. After another centrifugation step we resuspended the pellet which now is a mixture of donor and recipient cells in 100 µl LB medium without antibiotics. We than transferred the cell suspension on a membrane filter, which was placed on LB plates before and incubated these plates at 37 °C for 0, 6, 12, 18, 24, 30, 36, 42 minutes. Afterwards the membrane was put into 1 ml LB medium and the cells were resuspended by vortexing for 30 seconds. The cell suspension was diluted 10-5 and plated on the LB / ampicillin + chloramphenicol (CA) plates. The plates were incubated at 37 °C overnight and on the third day the colonies could be counted.
We have done this test thrice independently and calculated the conjugation efficient which is defined as [transconjugants per ml] / [donor per ml] from this assay.
To count the donor and recipient cell density small aliquots of the used suspensions were diluted 10-7 and plated on agar plates containing kanamycin + ampicillin for donor cells and chloramphenicol for the recipients.
Table 1: Conjugation kinetics of oriT BBa_J01003 between E. coli Top10.
| Group | Test A | Test B | Test C | |||
| Cell | Donor | Recipient | Donor | Recipient | Donor | Recipient |
| Strain | Top10 | Top10 | Top10 | Top10 | Top10 | Top10 |
| Cell number | 9325 | 8100 | 3465 | 8330 | 7140 | 7470 |
| Time [min] | Transconjugants | Efficiency | Transconjugants | Efficiency | Transconjugants | Efficiency |
| 0 | 150 | 0.016 | 9 | 0,003 | 37 | 0,005 |
| 6 | 500 | 0.054 | 20 | 0,006 | 390 | 0,055 |
| 12 | 780 | 0.084 | 94 | 0,027 | 1390 | 0,195 |
| 18 | 1160 | 0.124 | 172 | 0,050 | 1720 | 0,241 |
| 24 | 1400 | 0.150 | 262 | 0,076 | 2880 | 0,403 |
| 30 | 3360 | 0.360 | 1450 | 0,418 | 3500 | 0,490 |
| 36 | 1790 | 0.517 | 3750 | 0,525 | ||
| 42 | 2170 | 0.626 | ||||
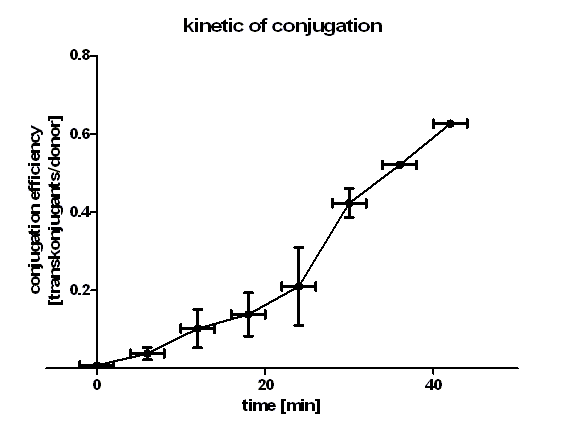
Figure 1 shows that the kinetic of the conjugative plasmid BBa_J01003 has three phases. In the first (0 min to 24 min) and in the third phase (30 min to 42 min) the curve has a smaller slope, whereas the second phase (24 min to 30 min) it has a very big slope. An explanation for this result could be that the cell growth in the first 24 min can be neglected due to the average generation time of E. coli in the LB medium which lies between 20 and 30 minutes whereas in the second phase the growth of E. coli dominates the conjugation process and leads to this deviation from linearity. In this test we used very concentrated bacteria suspension in a small conjugation volume which leads to the observation of conjugation in an approximately linear process in the primary phase.
We have done a linear regression analysis for the first phase (0 min to 24 min) and we calculated the slope for this phase as 9.00 • 10-3 [transconjugants • donor-1 • minute -1]. We calculated the conjugation rate for BBa_J01003 which is defined as transconjugants • donor-1 • recipient-1 • minute -1. The conjugation rate of BBa_J01003 is 1.05 • 10-12 [ml • min-1 • cell-1]. This rate was used by the modeling group as a parameter value.
Figure 1 also shows us that the transport of the plasmid pSB1A2 containing the part BBa_J01003 by conjugation is a very fast process since we got transconjugants in the first minutes of the experiment. Through this experiment we can conclude that the transport of the plasmid pSB1A2 containing BBa_J01003 by conjugation needs only about 2 minutes.
Since we have two conjugative plasmids in our donor cells, we wanted to see which plasmid is preferred to be transported by conjugation. We therefore plated the cells in the test C (see table 2) also on agar plates containing chloramphenicol + kanamycin (CK) and chloramphenicol + kanamycin + ampicillin (CKA) to select for transconjugants getting pUB307 and both plasmids.
Table 2: Test C conjugation kinetics of oriT BBa_J01003 and pUB307 between E. coli Top10.
| Select | Chlorophencol+Kanamycin (J01103) | Chlorophencol+Kanamycin (pUB307) | Chlorophenicol+Ampicilin+Kanamycin | |||
| Cell | Donor | Recipient | Donor | Recipient | Donor | Recipient |
| Strain | Top10 | Top10 | Top10 | Top10 | Top10 | Top10 |
| Cell number | 7140 | 7470 | 7140 | 7470 | 7140 | 7470 |
| Time [min] | Transconjugants | Efficiency | Transconjugants | Efficiency | Transconjugants | Efficiency |
| 0 | 37 | 0.005 | 2 | 0,000 | 1 | 0,000 |
| 6 | 390 | 0.055 | 17 | 0,002 | 4 | 0,001 |
| 12 | 1390 | 0.195 | 194 | 0,027 | 90 | 0,013 |
| 18 | 1720 | 0.241 | 230 | 0,032 | 109 | 0,015 |
| 24 | 2880 | 0.403 | 1130 | 0,158 | 800 | 0,112 |
| 30 | 3500 | 0.490 | 2500 | 0,350 | 1540 | 0,216 |
| 36 | 3750 | 0.525 | 2600 | 0,364 | 1800 | 0,252 |
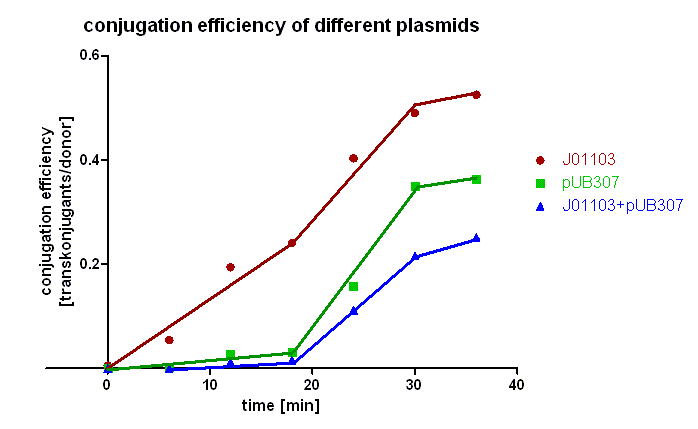
Figure 2 clearly shows that the plasmid pSB1A2 containing only BBa_J01003 is preferably transported during the first 20 minutes in comparison to pUB307. Since pUB307 has the same oriT as BBa_J01003 is, we assume the size of the plasmid to be the reason for this since BBa_J01003 in pSB1A2 is approx. 2.5 kb in size whereas pUB307 has 60 kb. A comparison of the red and the green curve shows us that the difference in the duration of the transport of plasmids with different size is not very big. Looking at the blue curve you can see that the first transconjugants with both plasmids can be detected after approx. 18 minutes which is longer than the sum of both transport times.
Test 2 Conjugation kinetics of oriT BBa_J01003 between different E.coli strains
For this experiment we used the same technique as described in test 1. In this case we used different E. coli strains as recipients, namely E. coli Top10, MG1655 and DH5α. In this experiment the cells on the membranes were incubated for 0, 6, 12, 18, 24, 30, 36 and 42 minutes at 37 °C. This test was conducted twice independently and the conjugation efficiency calculated as described in test 1.
Table 3: Conjugation kinetics of oriT BBa_J01004 between different E. coli strains.
| Group | Test D (Donor: Top10; Cell number: 5440) | Test E (Donor: Top10; Cell number: 7140) | ||||
| Cell | Recipient | Recipient | Recipient | Recipient | Recipient | Recipient |
| Strain | Top10 | MG1655 | DH5alpha | Top10 | MG1655 | DH5alpha |
| Cell number | 6086 | 5566 | 5096 | 7470 | 7275 | 6900 |
| Time [min] | Efficiency | Efficiency | Efficiency | Efficiency | Efficiency | Efficiency |
| 0 | 0.002 | 0,000 | 0,000 | 0,005 | 0,000 | 0,001 |
| 6 | 0.002 | 0,000 | 0,000 | 0,055 | 0,000 | 0,003 |
| 12 | 0.003 | 0,000 | 0,000 | 0,195 | 0,001 | 0,067 |
| 18 | 0.005 | 0,000 | 0,001 | 0,241 | 0,007 | 0,165 |
| 24 | 0.021 | 0,000 | 0,018 | 0,403 | 0,019 | 0,269 |
| 30 | 0.071 | 0,001 | 0,075 | 0,490 | 0,036 | 0,415 |
| 36 | 0.136 | 0,013 | 0,246 | 0,525 | 0,129 | 0,482 |
| 42 | 0.041 | 0,301 | 0,134 | 0,566 | ||
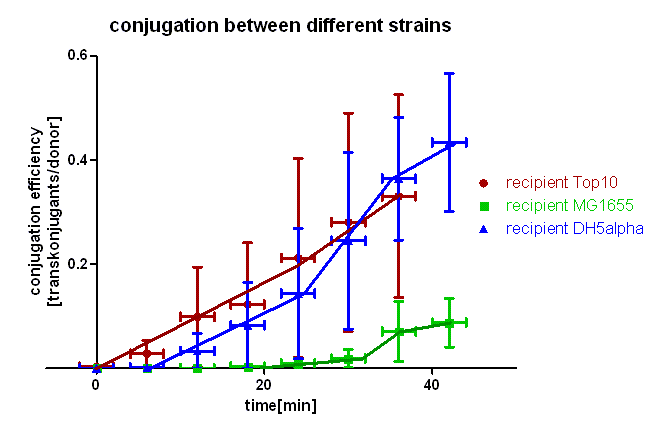
As you can see in figure 3 there is a difference in the conjugation kinetic between Top10 as donor and different strains as recipient. With E. coli Top10 as donor E. coli DH5α is a comparably good recipient as Top10 whereas MG1655 showed up as a bad recipient under these conditions. Comparing the red with the blue curve one can see that DH5α transconjugants appear later (6 minutes) that Top10, but the conjugation efficiency and also the conjugation rate (slope between 6 and 24 minutes) are in the same range with Top10 and DH5α. We assume that more time is needed for stabilizing the pillus contact between Top10 and DH5α than between two Top10 cells, but the conjugation between Top10 and DH5α can be done as good as between two Top10 cells. Comparing the red and the green curve one can see that the first MG1655 transconjugants appear much later (20 minutes) and the conjugation efficiency and rate (slope between 20 and 32 minutes) are much smaller. Therefore we conclude that conjugation between different strains is possible using this oriT but the efficiency depends on the used strains and their compatibility.
Test 3 Conjugative competence of oriT BBa_J01003 at different donor/recipient ratios
In this experiment we again used the same protocol as described above in test 1 but used different donor / recipient rations, namely 50:1, 25:1, 10:1, 8:1, 5:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:5, 1:10, 1:25, and 1:50. This test was conducted twice independently and the conjugation efficiency calculated as described above. The donor and recipient cell density was measured by plating 10-7 dilutions on agar plates containing kanamycin + ampicillin for the donor and chloramphenicol for recipient cells.
Table 4: Conjugative competence of otiT BBa_J01003 at different donor / recipient ratios.
| Group | Test F | Test G | ||||||
| Anticipant ratio | Donor | Recipient | Actual ratio | Transconjugants | Donor | Recipient | Actual ratio | Transconjugants |
| 50:1 | 1003 | 23 | 43:1 | 12 | 832 | 21 | 40:1 | 16 |
| 25:1 | 982 | 46 | 21.5:1 | 28 | 815 | 41 | 20:1 | 31 |
| 10:1 | 930 | 110 | 8.5:1 | 62 | 729 | 97 | 7.5:1 | 63 |
| 8:1 | 909 | 133 | 7:1 | 81 | 711 | 117 | 6:1 | 75 |
| 5:1 | 854 | 202 | 4:1 | 115 | 665 | 153 | 4.5:1 | 120 |
| 3:1 | 767 | 302 | 2.5:1 | 161 | 599 | 266 | 2.3:1 | 129 |
| 2:1 | 680 | 403 | 1.7:1 | 213 | 533 | 355 | 1.5:1 | 212 |
| 1:1 | 510 | 605 | 1:1.2 | 302 | 400 | 532 | 1:1.3 | 303 |
| 1:2 | 340 | 802 | 1:2.3 | 351 | 268 | 705 | 1:2.6 | 298 |
| 1:3 | 257 | 902 | 1:3.5 | 294 | 199 | 794 | 1:4 | 302 |
| 1:5 | 170 | 1003 | 1:5.9 | 289 | 133 | 883 | 1:6.7 | 194 |
| 1:8 | 122 | 1072 | 1:8.8 | 241 | 89 | 943 | 1:10.5 | 190 |
| 1:10 | 94 | 1095 | 1:11.7 | 158 | 72 | 963 | 1:13 | 134 |
| 1:25 | 40 | 1159 | 1:29 | 86 | 31 | 1020 | 1:33 | 82 |
| 1:50 | 20 | 1182 | 1:59 | 31 | 16 | 1040 | 1:67 | 30 |
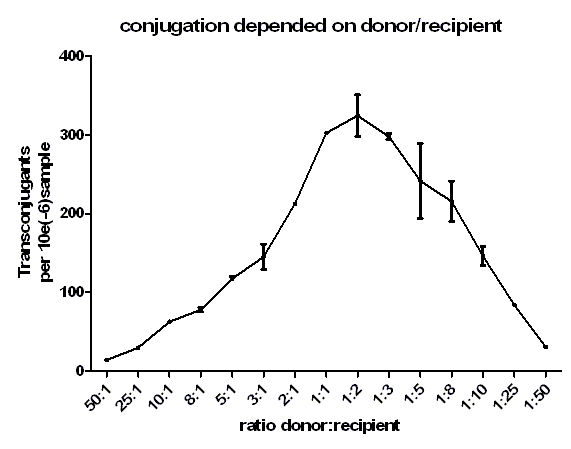
Figure 4 shows very clearly that at same cell density, if donor : recipient ratio is 1 : 2 the highest amount of transconjugants can be achieved. A big difference in the amount of donor : recipient leads to a low number of transconjugants. At low donor : recipient ratios the conjugation efficiency was quiet high but lead to a low number of transconjugants since the density of donor cells was too low. Only at donor : recipient rations between 1 : 1 and 1 : 3, a high conjugation efficiency and a high donor density was achieved leading to a high number of transconjugants. A maximum in the number of transconjugants could be achieved at a ratio of 1 : 2.
Test 4 Conjugative competence of oriT BBa_J01003 at different temperatures
In this experiment the same method is used as described above in test 1. Here, donor and recipient both were E. coli Top10. The incubation during conjugation took place at different temperatures, namely 4 °C, 22 °C, 30 °C, 37 °C and 42 °C for 20, 40 and 60 minutes. For counting of transconjugants the cell suspension was diluted 10-6 and plated on agar containing ampicillin and chloramphenicol (CA). This experiment was conducted twice independently. The donor and recipient cell density was again calculated by plating 10-7 dilutions on plates containing kanamycin and ampicillin or chloramphenicol, respectively.
Table 5: Conjugative competence of otiT BBa_J01003 at different temperatures.
| Group | Test H | Test J | ||||
| Cell | Donor | Recipient | donor : recipient | Donor | Recipient | donor/recipient |
| Strain | Top10 | Top10 | 1 : 2.02 | Top10 | Top10 | 1:1.77 |
| Cell number | 361 | 731 | sum:1092 | 330 | 584 | sum:914 |
| Sample | Transconjugants | Transconjugants | ||||
| Temperature | 10min | 40min | 60min | 20min | 40min | 60min |
| 4°C | 5 | 5 | 1 | 2 | 1 | 0 |
| 22°C | 2 | 78 | 117 | 29 | 61 | 79 |
| 30°C | 71 | 178 | 241 | 43 | 117 | 227 |
| 37°C | 154 | 285 | 550 | 118 | 290 | 332 |
| 42°C | 167 | 273 | 402 | 122 | 271 | 234 |
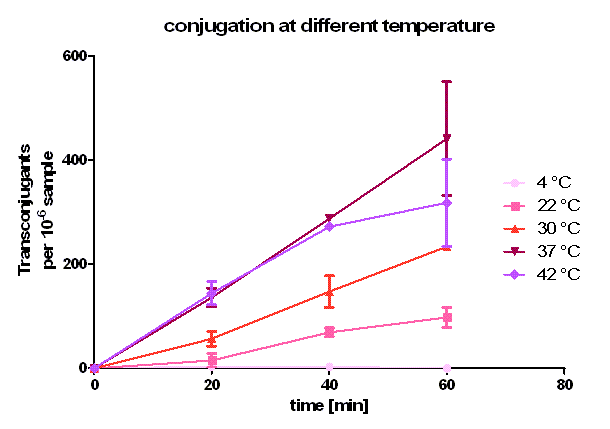
As you can see in figure 5, conjugation is most efficient at 37 °C. From 0 to 20 minutes during the primary conjugation phase the cell growth can be neglected. From 20 to 40 minutes, the cell growth is also presented in the conjugation curves. At 4 °C, the conjugation curve has a very small slope (near zero) and no significant transconjugants production could be observed. At 22, 30, 37 and 42 °C, a significant number of transconjugants could be achieved, but the conjugation efficiency and the conjugation rate (slope between 0 and 20 minutes) differ strongly. From 22 °C to 37°C, the conjugation efficiency and conjugation rate increases with rising temperature. Comparing these three curves between 20 and 40 minutes one can see that the cell growth gets more important with rising temperature. Dynamically, the cells have more mobility at higher temperature, and have a bigger possibility to touch another cell, which is necessary for conjugation. When we compared the conjugation curve at 42 °C with the one at 37 °C, one can see, that the conjugation efficient and the conjugation rate at 42 °C is as good as at 37 °C. After the primary conjugation phase, the production of transconjugants at 42 °C is clearly worse than the production at 37 °C what can be seen at comparing the curves between 40 and 60 minutes. A possible reason for this could be a lower growth and higher death rate at 42 °C compared to 37 °C. So, one can conclude, that for best conjugation efficiency one needs a warm environment for the conjugation to take place but also temperatures which favor cell growth.
Summary
The characterization of BBa_J01003 in pSB1A2 has given the following results:
- The conjugation has an average conjugation rate of 1.05 • 10-12 [ml • min-1 • cell-1]
- The conjugation has an approximate transport time of 2 minutes
- The oriT BBa_J01003 will be transported first in attendance of pUB307 in the donor cells
- Smaller plasmids favor the conjugation and are conjugated first
- The oriT BBa_J01003 can be conjugated between Top10 cells and from Top10 to DH5α cells with similar efficiency but from Top10 to MG1655 with a strongly lower efficiency
- The conjugation favors a donor : recipient ratio of 1 : 2
- The conjugation has the best production of transconjugants, the best efficiency and the best conjugation rate at 37 °C
User Reviews
UNIQa0a228ab554ccf3f-partinfo-00000000-QINU UNIQa0a228ab554ccf3f-partinfo-00000001-QINU
