Difference between revisions of "Part:BBa I764001"
| Line 14: | Line 14: | ||
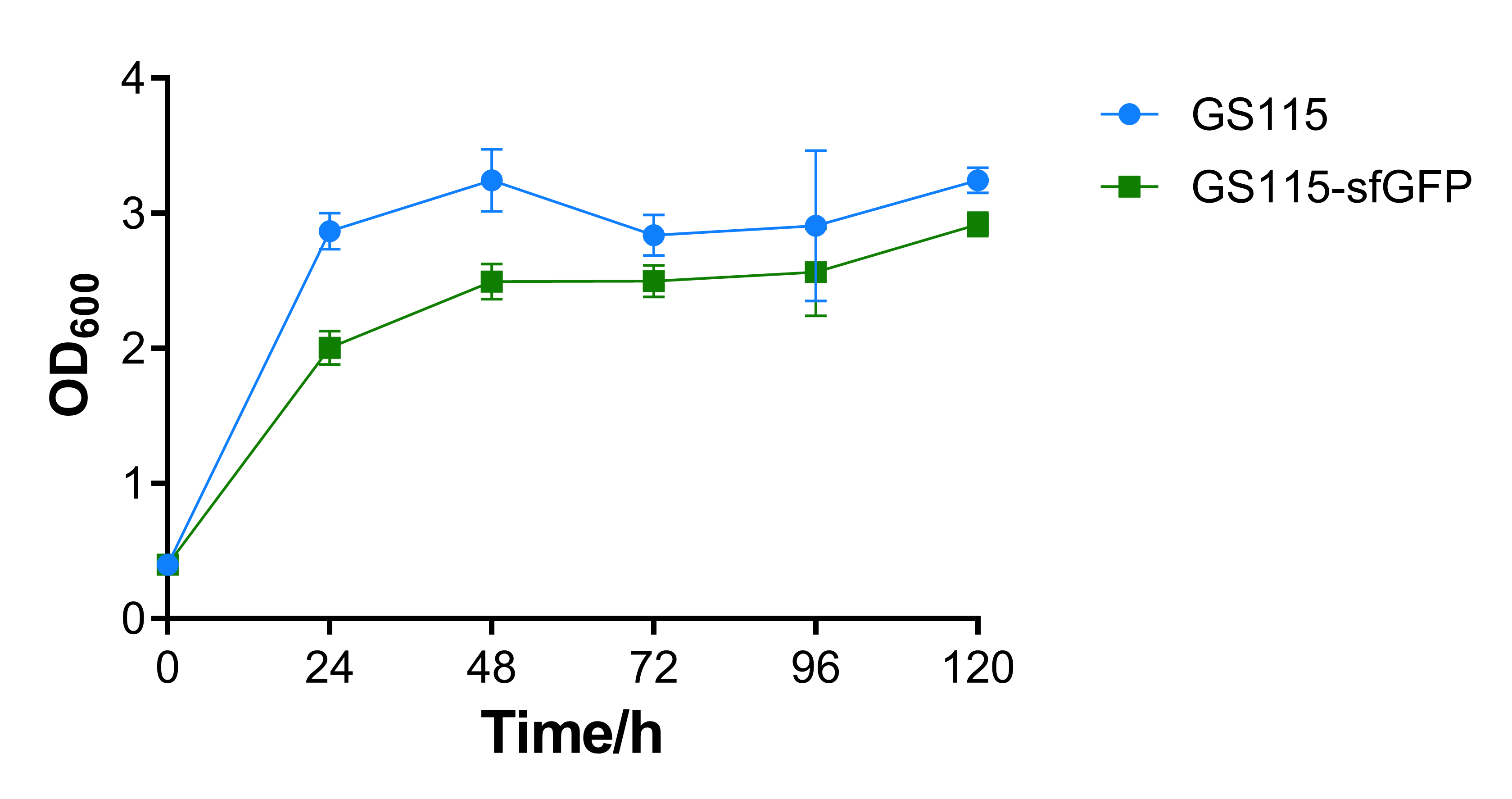
We added sfGFP gene behind AOX1 promoter, inserted the whole sequence into pPIC9K backbone and transformed the plasmid into P. pastoris GS115. The recombinant strain was conducted fermentation test BMMY Medium. We measured the OD600 absorbance and fluorescence of the fermentation broth every 24 hours, and the supernatant samples during the fermentation were verified through SDS-PAGE gel electrophoresis. | We added sfGFP gene behind AOX1 promoter, inserted the whole sequence into pPIC9K backbone and transformed the plasmid into P. pastoris GS115. The recombinant strain was conducted fermentation test BMMY Medium. We measured the OD600 absorbance and fluorescence of the fermentation broth every 24 hours, and the supernatant samples during the fermentation were verified through SDS-PAGE gel electrophoresis. | ||
| − | <b>Growth curve and fluorescence test<b> | + | <b>Growth curve and fluorescence test</b> |
The OD600 absorbance of the recombinant strain that contains sfGFP is a little lower than the control strain P. pastoris GS115. That may be caused by the additional expression of sfGFP, the results show that expression of heterologous gene would repress cell growth, while the repression is not intensive. | The OD600 absorbance of the recombinant strain that contains sfGFP is a little lower than the control strain P. pastoris GS115. That may be caused by the additional expression of sfGFP, the results show that expression of heterologous gene would repress cell growth, while the repression is not intensive. | ||
| − | [[File:T-- | + | [[File:T--BEIJING 4ELEVEN--Contribution Figure 1 OD600.png|600px|thumb|center|Figure 1. OD600 absorbance of P. pastoris GS115 and recombinant strain that contains sfGFP every 24 hours.]] |
Revision as of 13:14, 23 October 2020
Ethanol regulated promoter AOX1
The AOX1 promoter region from Pichia pastoris.
A complex pathway for the metabolism of methanol exists within some species of the Pichia genus. Alcohol oxidase (AO) appears to be the first and major enzyme produced in this metabolic pathway (1). Transcribed from its gene (AOX1), AO converts methanol to formaldehyde within the yeast’s peroxisome (1). A metabolic pathway for the utilization of ethanol is also present within the yeast. However, if both ethanol and methanol is present, the yeast will utilize the ethanol before consuming the methanol (2). Consequently, the AOX gene will not be expressed to produce the AO enzyme until the ethanol has been consumed.
Performance of AOX1 in in Pichia pastoris
Characterized by Beijing_4ELEVEN 2020
In our project, we choose AOX1 promoter (BBa_I764001), which is a common inducible promoter used in Pichia pastoris GS115, to control the expression. To make sure the AOX1 promoter works properly, we tested its performance with sfGFP.
We added sfGFP gene behind AOX1 promoter, inserted the whole sequence into pPIC9K backbone and transformed the plasmid into P. pastoris GS115. The recombinant strain was conducted fermentation test BMMY Medium. We measured the OD600 absorbance and fluorescence of the fermentation broth every 24 hours, and the supernatant samples during the fermentation were verified through SDS-PAGE gel electrophoresis.
Growth curve and fluorescence test
The OD600 absorbance of the recombinant strain that contains sfGFP is a little lower than the control strain P. pastoris GS115. That may be caused by the additional expression of sfGFP, the results show that expression of heterologous gene would repress cell growth, while the repression is not intensive.
1. Cregg, James M., K. R. Madden, K. J. Barringer, G. P Thill, and C. A. Stillman. 1989. Functional Characterization of the Two Alcohol Oxidase Genes from the Yeast Pichia pastoris. Molecular and Cellular Biology. 9:1316-1323.
2. Inan Mehmet and Michael M. Meagher. The Effect of Ethanol and Acetate on Protein Expression in Pichia pastoris. 2001. Journal of Bioscience and Bioengineering. 9: 337-341.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]