Difference between revisions of "Part:BBa K1471000"
| (One intermediate revision by the same user not shown) | |||
| Line 25: | Line 25: | ||
<html> | <html> | ||
<center><img src="http://i1088.photobucket.com/albums/i325/mggo93/imagemerE2.png?t=1414699634" style="width: 700px;"></center> </html> | <center><img src="http://i1088.photobucket.com/albums/i325/mggo93/imagemerE2.png?t=1414699634" style="width: 700px;"></center> </html> | ||
| + | |||
| + | ==MIT_MAHE 2020== | ||
| + | |||
| + | '''Role of cysteine and histidine residues.''' | ||
| + | |||
| + | In order to investigate the molecular function of merE in the transport of methylmercury, a study was conducted with six merE variants with specific site-directed mutations. By comparison methylmercury uptake by cell with intact merE and mutated MerE, it was demonstrated that the cysteine pairs (Cys28 and Cys30) within the first transmembrane domain of MerE and the histidine residue (His51) on the periplasmic face located between the first and second transmembrane domains were required for MerE mediated CH3Hg(I) transport across the cell membrane. The cysteine pairs especially may play a critical role in the transport of CH3Hg(I) and Hg(II). However, it is still unclear why the merE gene found in the T21 mer operon, confers resistance only to Hg(II) and not CH3Hg(I). | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 46: | Line 52: | ||
Kiyono M., Sone Y., Nakamura R., ''et al'' (2013) ''Role of MerC, MerE, MerF, MerT, and/or MerP in Resistance to Mercurials and the Transport of Mercurials in Escherichia coli''. Biological and Pharmaceutical Bulletin. Volume 36; Issue 11; pages 1835-1841 | Kiyono M., Sone Y., Nakamura R., ''et al'' (2013) ''Role of MerC, MerE, MerF, MerT, and/or MerP in Resistance to Mercurials and the Transport of Mercurials in Escherichia coli''. Biological and Pharmaceutical Bulletin. Volume 36; Issue 11; pages 1835-1841 | ||
| + | |||
| + | Sone, Y., Uraguchi, S., Takanezawa, Y., Nakamura, R., Pan-Hou, H., & Kiyono, M. (2017). Cysteine and histidine residues are involved in Escherichia coli Tn21 MerE methylmercury transport. FEBS open bio, 7(12), 1994–1999. https://doi.org/10.1002/2211-5463.12341 | ||
Latest revision as of 17:14, 19 October 2020
MerE.
For mercury bioaccumulation.
Brief description merE
Our part is a yeast RBS with an 8KDa CH3Hg or Hg+2 transmembrane bacterial transporter.
Biology
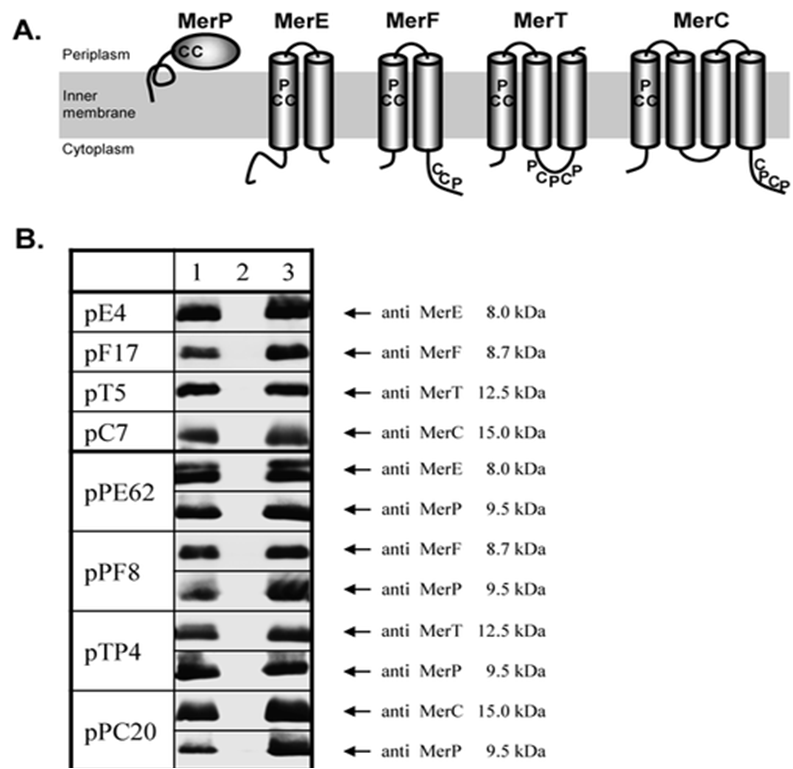
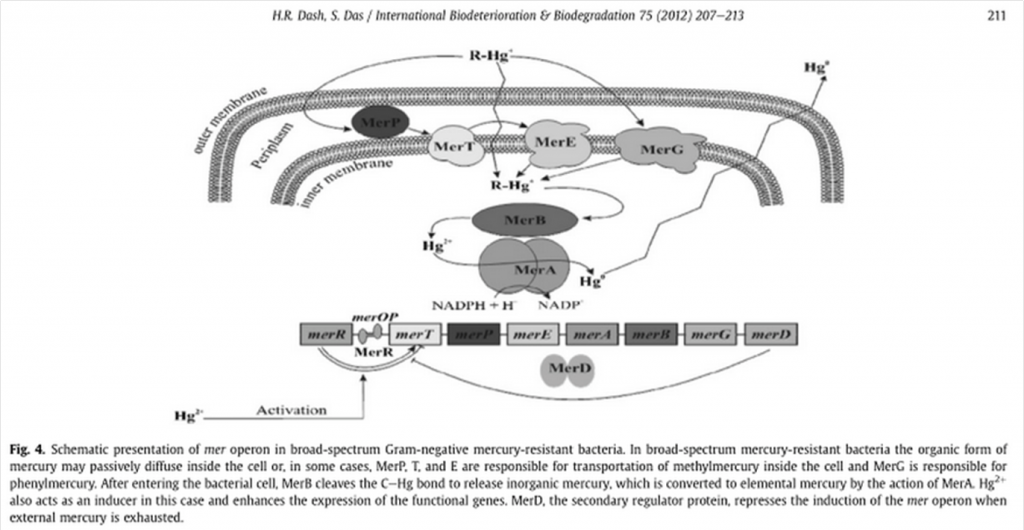
MerE is a gene is part of the mer operon, a collection of bacterial genes specialized on the tolerance to various compounds of mercury including methylmercury. It is naturally found in the transposon Tn21 from the plasmid NR1 Shigella flexneri or MB1 in the case of Bacillus megaterium. The general mechanism of the operon can be observed on the following representation (Das S., Dash H. R., 2012):
Schematic presentation of mer operon in narrow-spectrum Gram-negative mercury-resistant bacteria (Das S., Dash H. R., 2012) Where organomercury compounds are transported inside the bacteria by merP, merT, mer E and merG, followed by the transformation of organic mercury by merB into its ionic form and the reduction from Hg+2 into volatile Hg0 by mer A with the help of NADPH(Das S., Dash H. R., 2012). Although the transportation of methylmercury is barely understood, there is evidence that recombinant E. coli and other transformed GRAM negative bacteria are able to accumulate mercury thanks to the transformation of organic mercury into its ionic form(Das S., Dash H. R., 2012).
Behaviour
merE has been characterized previously on A. thaliana as a potential mercury accumulator and transporter. In Kyono,M., et al (2013)´s study shoot and root growth were observed to become more tolerant in transgenic Arabidopsis compared with controls. As it can be seen on the figure:
MIT_MAHE 2020
Role of cysteine and histidine residues.
In order to investigate the molecular function of merE in the transport of methylmercury, a study was conducted with six merE variants with specific site-directed mutations. By comparison methylmercury uptake by cell with intact merE and mutated MerE, it was demonstrated that the cysteine pairs (Cys28 and Cys30) within the first transmembrane domain of MerE and the histidine residue (His51) on the periplasmic face located between the first and second transmembrane domains were required for MerE mediated CH3Hg(I) transport across the cell membrane. The cysteine pairs especially may play a critical role in the transport of CH3Hg(I) and Hg(II). However, it is still unclear why the merE gene found in the T21 mer operon, confers resistance only to Hg(II) and not CH3Hg(I).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
Das S., Dash H. R., (2012). Bioremediation of mercury and the importance of bacterial mer genes. National Institute of Technology.India: International Biodeterioration & Biodegradation. Volume 75. Pages 207-213
Kiyono M. , et al (2013) Increase methylmercury accumulation in Arabidopsis thaliana expressing bacterial broad-spectrum mercury transporter MerE. Springer. Issue 3; Pages 1-13
Kiyono M., Sone Y., Nakamura R., et al (2013) Role of MerC, MerE, MerF, MerT, and/or MerP in Resistance to Mercurials and the Transport of Mercurials in Escherichia coli. Biological and Pharmaceutical Bulletin. Volume 36; Issue 11; pages 1835-1841
Sone, Y., Uraguchi, S., Takanezawa, Y., Nakamura, R., Pan-Hou, H., & Kiyono, M. (2017). Cysteine and histidine residues are involved in Escherichia coli Tn21 MerE methylmercury transport. FEBS open bio, 7(12), 1994–1999. https://doi.org/10.1002/2211-5463.12341
