Difference between revisions of "Part:BBa K3142010"
(→Expression of GST-AHPM in E. coli BL21) |
(→Construction and Identification of Recombinant Expression Vector pGEX-4T-2-AHPM) |
||
| (16 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3142010 short</partinfo> | <partinfo>BBa_K3142010 short</partinfo> | ||
| − | + | The AHPM is composed of high activity hypotensive peptides VY, IPP, VPP, KYLCY and FKGKYYP repeated three times in series. The AHPM containing 78 amino acids was joined by arginine linker (FR), which is cleavage sites of common enzymes in the intestine, trypsin and α-chymotrypsin. Therefore, AHPM can be hydrolyzed by intestinal hydrolase to produce a large number of active hypotensive peptides. | |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 30: | Line 30: | ||
*Phase B is 0.1% TFA with 5% ACN. | *Phase B is 0.1% TFA with 5% ACN. | ||
Chromatographic conditions: flow rate 0.8ml / min, column temperature 30 ° C, elution gradient 30% ~ 60% (A phase) 30min, hip uric acid retention time is 4.1min. | Chromatographic conditions: flow rate 0.8ml / min, column temperature 30 ° C, elution gradient 30% ~ 60% (A phase) 30min, hip uric acid retention time is 4.1min. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Construction of the antihypertensive peptides multimer (AHPM)== | ==Construction of the antihypertensive peptides multimer (AHPM)== | ||
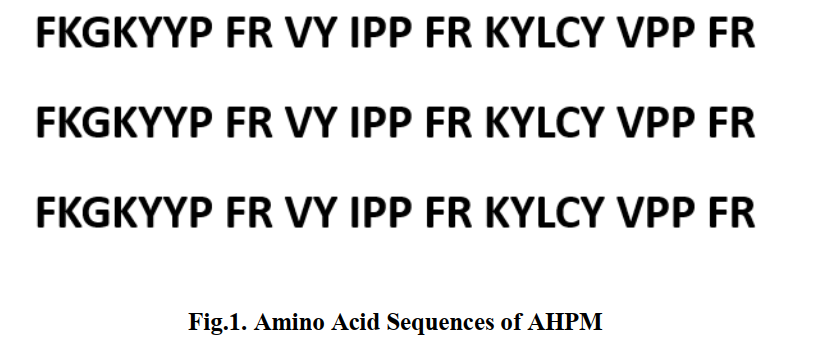
| − | Since AHPs are composed of only a few amino acids, it is difficult to directly biosynthesize. The newly designed high activity hypotensive peptides KYLCY and FKGKYYP and fully validated VY, IPP and VPP selected from the database were used to construct AHPM. The AHPM containing 78 amino acids was joined by FR, which is cleavage sites of common enzymes in the intestine, trypsin and α-chymotrypsin. The amino acid sequence of AHPM is shown in Fig. | + | Since AHPs are composed of only a few amino acids, it is difficult to directly biosynthesize. The newly designed high activity hypotensive peptides KYLCY and FKGKYYP and fully validated VY, IPP and VPP selected from the database were used to construct AHPM. The AHPM containing 78 amino acids was joined by FR, which is cleavage sites of common enzymes in the intestine, trypsin and α-chymotrypsin. The amino acid sequence of AHPM is shown in Fig.1. |
| − | [[File: | + | [[File:new1.png|500px|center]] |
| + | |||
==Fusion expression of AHPM in ''E. coli'' system== | ==Fusion expression of AHPM in ''E. coli'' system== | ||
===Construction and Identification of Recombinant Expression Vector pGEX-4T-2-AHPM=== | ===Construction and Identification of Recombinant Expression Vector pGEX-4T-2-AHPM=== | ||
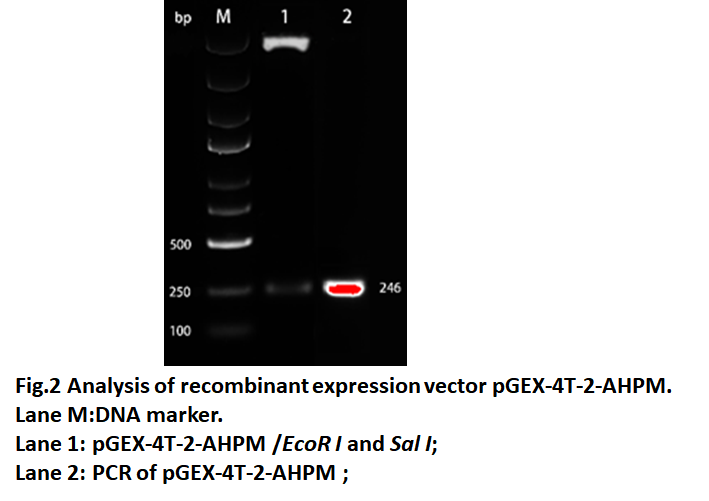
| − | The AHPM gene was cloned into the pGEX-4T-2 expression vector by ''EcoRⅠ'' and ''SalⅠ'' restriction enzyme sites in the 5' and 3' regions, respectively. Positive clones from LB plates containing 100 μg/mL ampicillin were selected for restriction analysis and PCR. As shown in Fig. | + | The AHPM gene was cloned into the pGEX-4T-2 expression vector by ''EcoRⅠ'' and ''SalⅠ'' restriction enzyme sites in the 5' and 3' regions, respectively. Positive clones from LB plates containing 100 μg/mL ampicillin were selected for restriction analysis and PCR. As shown in Fig.2, Lane 1 and lane 2 both have fragments of 246 bp, which is the same as AHPM. The result show that the recombinant expression vector named pGEX-4T-2-AHPM was constructed successfully. |
| − | [[File: | + | [[File:20191022112006.png|500px|center]] |
| − | ===Expression of GST-AHPM in ''E. coli | + | |
| − | The recombinant strain ''E. coli BL21 (DE3) | + | ===Expression of GST-AHPM in ''E. coli'' BL21=== |
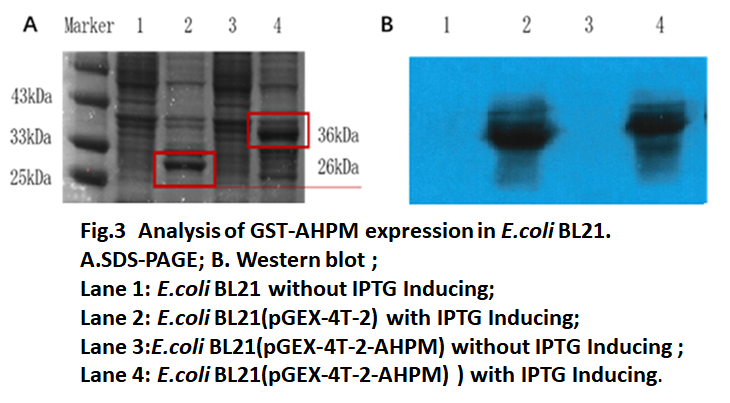
| − | [[File: | + | The recombinant strain ''E. coli'' BL21 (DE3) with pGEX-4T-2-AHPM vector was induced by IPTG for 6 hours and then collected for SDS-PAGE and Western blot analysis. The results of SDS-PAGE were shown in Fig.3. After IPTG induction, the ''E. coli'' BL21 transferred with pGEX-4T-2 vector produced 26kDa GST protein (lane2) and ''E. coli'' BL21 transferred with pGEX-4T-2-AHPM produced 36kDa protein matched well with our GST-AHPM fusion protein (lane 4). In contrast, Non-induced ''E. coli'' BL21 cells did not express GST or GST-AHPM (lane 1, lane3). Western blot results showed that 26kDa GST protein and 36kDa fusion protein carry GST tag (Fig 4B). The results showed that the constructed tandem AHP could be expressed in the fusion expression system. |
| + | [[File:20191022111804.png|500px|center]] | ||
===In Vitro Activity of AHPM=== | ===In Vitro Activity of AHPM=== | ||
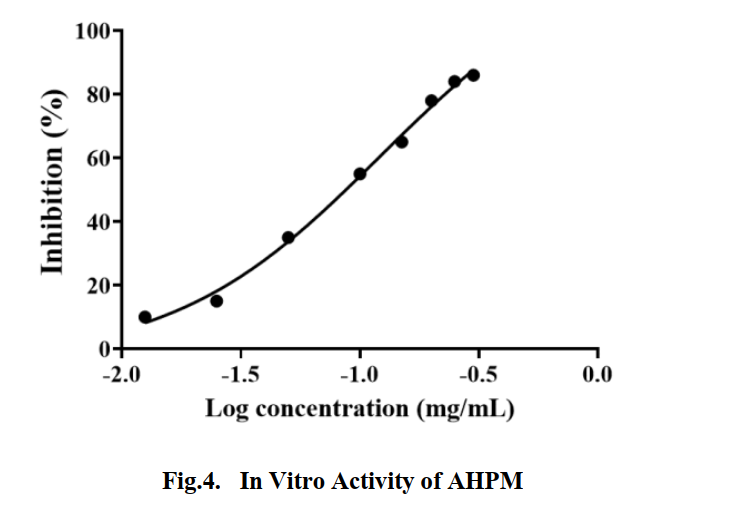
| − | The fusion protein GST- | + | The fusion protein GST-AHPM was purified by GST affinity chromatography. After trypsin and α-chymotrypsin digestion, the GST protein was removed by GST affinity filler and then the polypeptide mixture was obtained. The activity of AHP multimer was determined in vitro. The in vitro activity of the mixed peptide is shown in the Fig.4. The IC50 of inhibiting ACE activity is 0.1210mg/ml. |
| − | [[File: | + | [[File:new4.png|500px|center]] |
| + | |||
| + | ===Reference=== | ||
| + | *Chen heru, Xu zhicheng.Reversed-phase HPLC Determination of the in-vitro Inhibition Kinetics of Angiotensin-converting Enzyme Inhibitors. JOURNAL OF INSTRUMENTAL ANALYSIS. 1999,18(1):69-71 | ||
| + | *ocksan I. Morales-Camacho, Edgar Espinosa-Hernández, F. Fátima Rosas-Cárdenas, et .al. Insertions of antihypertensive peptides and their applications in pharmacy and functional foods. Applied Microbiology and Biotechnology.2019,103(6):2493-2505 | ||
Latest revision as of 03:22, 22 October 2019
Antihypertensive peptide multimer(AHPM)
The AHPM is composed of high activity hypotensive peptides VY, IPP, VPP, KYLCY and FKGKYYP repeated three times in series. The AHPM containing 78 amino acids was joined by arginine linker (FR), which is cleavage sites of common enzymes in the intestine, trypsin and α-chymotrypsin. Therefore, AHPM can be hydrolyzed by intestinal hydrolase to produce a large number of active hypotensive peptides.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
In vitro activity detection of antihypertensive peptide(HPLC)
Principle
ACE can catalyze the decomposition of HHL to produce hippuric acid, while the blood pressure lowering peptide reduces the production of hippuric acid by inhibiting ACE activity, and the absorption of hippuric acid at 228 nm is determined by reverse phase HPLC to calculate the inhibition rate of the polypeptide of interest.
Method
Add 1.667×10-6moL/(s ∙ kg) ACE 25μL to the sample tube, normal tube and blank tube, respectively, 0.1 mol/L boric acid-borax buffer solution with a pH value of 8.3, 160, 170, 170 μL Add 10uL of blood pressure lowering peptide sample to the sample tube, and the other two tubes are not added and mixed. The blank tube was sterilized for 5 min at 100 ° C, and the other two tubes were reacted at 37 ° C for 5 min. Add 5 μL of HHL substrate to each tube, the total volume is 200 μL, and react at 37 ° C for 30 min. 100 ° C inactivated enzyme. Mobile phase composition:
- Phase A is 100% ACN plus 0.1% TFA
- Phase B is 0.1% TFA with 5% ACN.
Chromatographic conditions: flow rate 0.8ml / min, column temperature 30 ° C, elution gradient 30% ~ 60% (A phase) 30min, hip uric acid retention time is 4.1min.
Construction of the antihypertensive peptides multimer (AHPM)
Since AHPs are composed of only a few amino acids, it is difficult to directly biosynthesize. The newly designed high activity hypotensive peptides KYLCY and FKGKYYP and fully validated VY, IPP and VPP selected from the database were used to construct AHPM. The AHPM containing 78 amino acids was joined by FR, which is cleavage sites of common enzymes in the intestine, trypsin and α-chymotrypsin. The amino acid sequence of AHPM is shown in Fig.1.
Fusion expression of AHPM in E. coli system
Construction and Identification of Recombinant Expression Vector pGEX-4T-2-AHPM
The AHPM gene was cloned into the pGEX-4T-2 expression vector by EcoRⅠ and SalⅠ restriction enzyme sites in the 5' and 3' regions, respectively. Positive clones from LB plates containing 100 μg/mL ampicillin were selected for restriction analysis and PCR. As shown in Fig.2, Lane 1 and lane 2 both have fragments of 246 bp, which is the same as AHPM. The result show that the recombinant expression vector named pGEX-4T-2-AHPM was constructed successfully.
Expression of GST-AHPM in E. coli BL21
The recombinant strain E. coli BL21 (DE3) with pGEX-4T-2-AHPM vector was induced by IPTG for 6 hours and then collected for SDS-PAGE and Western blot analysis. The results of SDS-PAGE were shown in Fig.3. After IPTG induction, the E. coli BL21 transferred with pGEX-4T-2 vector produced 26kDa GST protein (lane2) and E. coli BL21 transferred with pGEX-4T-2-AHPM produced 36kDa protein matched well with our GST-AHPM fusion protein (lane 4). In contrast, Non-induced E. coli BL21 cells did not express GST or GST-AHPM (lane 1, lane3). Western blot results showed that 26kDa GST protein and 36kDa fusion protein carry GST tag (Fig 4B). The results showed that the constructed tandem AHP could be expressed in the fusion expression system.
In Vitro Activity of AHPM
The fusion protein GST-AHPM was purified by GST affinity chromatography. After trypsin and α-chymotrypsin digestion, the GST protein was removed by GST affinity filler and then the polypeptide mixture was obtained. The activity of AHP multimer was determined in vitro. The in vitro activity of the mixed peptide is shown in the Fig.4. The IC50 of inhibiting ACE activity is 0.1210mg/ml.
Reference
- Chen heru, Xu zhicheng.Reversed-phase HPLC Determination of the in-vitro Inhibition Kinetics of Angiotensin-converting Enzyme Inhibitors. JOURNAL OF INSTRUMENTAL ANALYSIS. 1999,18(1):69-71
- ocksan I. Morales-Camacho, Edgar Espinosa-Hernández, F. Fátima Rosas-Cárdenas, et .al. Insertions of antihypertensive peptides and their applications in pharmacy and functional foods. Applied Microbiology and Biotechnology.2019,103(6):2493-2505