Difference between revisions of "Part:BBa K2959010"
(→References) |
|||
| (64 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
| − | <partinfo>BBa_K2959010 short</partinfo> | + | <partinfo>BBa_K2959010 short</partinfo><br> |
<p align="justify"> | <p align="justify"> | ||
This composite part consists of a T7 promoter, ribosome binding site, a coding sequence for AtPFN1 as a fusion protein with a 6x His-Tag, and a double terminator. This construct allows the expression of AtPFN, an antifungal peptide from <i>Arabidopsis thaliana</i> in <i>E. coli</i> BL21 (DE3). Expression can be positively regulates by the addition of IPTG thanks to its promoter. The part is designed to code for a fusion protein of AtPFN1 with a polyhistidine tag (6x His-Tag) at its C-terminus for purification by immobilized metal affinity chromatography. | This composite part consists of a T7 promoter, ribosome binding site, a coding sequence for AtPFN1 as a fusion protein with a 6x His-Tag, and a double terminator. This construct allows the expression of AtPFN, an antifungal peptide from <i>Arabidopsis thaliana</i> in <i>E. coli</i> BL21 (DE3). Expression can be positively regulates by the addition of IPTG thanks to its promoter. The part is designed to code for a fusion protein of AtPFN1 with a polyhistidine tag (6x His-Tag) at its C-terminus for purification by immobilized metal affinity chromatography. | ||
| Line 13: | Line 12: | ||
The binding affinity of antifungal proteins to fungal cells is the most important attribute for their fungal action, even if the mechanism is membranolytic or cell damaging. It has also been demonstrated that these proteins can be transferred across the cell membrane into the cytosolic space and accumulate in the cytosol of the cell by altering the membrane integrity. For cytosolic translocation the mechanisms used by the proteins are direct penetration, vacuolar localization and expansion, partial plasma membrane disruption, transition pore formation and endocytosis. AtPFN1 has exhibited a potent antifungal activity against fungal strains of <i>C. gloesporioides</i>, <i>F. osysporum</i>, <i>C. albicans</i>, and <i>C. glabrata</i><sup>2</sup>.</p> | The binding affinity of antifungal proteins to fungal cells is the most important attribute for their fungal action, even if the mechanism is membranolytic or cell damaging. It has also been demonstrated that these proteins can be transferred across the cell membrane into the cytosolic space and accumulate in the cytosol of the cell by altering the membrane integrity. For cytosolic translocation the mechanisms used by the proteins are direct penetration, vacuolar localization and expansion, partial plasma membrane disruption, transition pore formation and endocytosis. AtPFN1 has exhibited a potent antifungal activity against fungal strains of <i>C. gloesporioides</i>, <i>F. osysporum</i>, <i>C. albicans</i>, and <i>C. glabrata</i><sup>2</sup>.</p> | ||
<br> | <br> | ||
| − | + | ==<b>Characterization of Expressible Arabidopsis thaliana Profilin 1</b>== | |
<p align= "justify"> | <p align= "justify"> | ||
Our DNA sequence AtPFN1, was synthesized by IDT®️ with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGene®️ software, we could model our ligated expression plasmid, and the final parts resulted in a sequence of 2,651bp. Thereupon, Escherichia coli BL21(DE3) was transformed by heat shock for following antibiotic selection of clones. | Our DNA sequence AtPFN1, was synthesized by IDT®️ with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGene®️ software, we could model our ligated expression plasmid, and the final parts resulted in a sequence of 2,651bp. Thereupon, Escherichia coli BL21(DE3) was transformed by heat shock for following antibiotic selection of clones. | ||
</p> | </p> | ||
| + | |||
<center>[[File:T--Tec-Chihuahua--AtPFN1ff.png|400px]]</center> | <center>[[File:T--Tec-Chihuahua--AtPFN1ff.png|400px]]</center> | ||
<center><b>Figure 1.</b> SnapGene®️ map of BioBrick BBa_K2959010.</center> | <center><b>Figure 1.</b> SnapGene®️ map of BioBrick BBa_K2959010.</center> | ||
| Line 23: | Line 23: | ||
<p align= "justify"> | <p align= "justify"> | ||
The next step was to amplify our BioBrick sequence through colony PCR performed upon our transformed cells to confirm the presence of our expression plasmid inside of our chassis. With the help of the specific forward Biobrick prefix [BBa_G1004] and the specific reverse Biobrick suffix [BBa_G1005], we were able to amplify our sequence exclusively. Through an agarose gel we confirmed the correct transformation. The PCR action from SnapGene®️ was used to predict the size of the amplified sequence which resulted in a size of 640 bp.</p> | The next step was to amplify our BioBrick sequence through colony PCR performed upon our transformed cells to confirm the presence of our expression plasmid inside of our chassis. With the help of the specific forward Biobrick prefix [BBa_G1004] and the specific reverse Biobrick suffix [BBa_G1005], we were able to amplify our sequence exclusively. Through an agarose gel we confirmed the correct transformation. The PCR action from SnapGene®️ was used to predict the size of the amplified sequence which resulted in a size of 640 bp.</p> | ||
| − | |||
<center>[[File:T--Tec-Chihuahua--atpfn.pcr.png|700px]] | <center>[[File:T--Tec-Chihuahua--atpfn.pcr.png|700px]] | ||
[[File:T--Tec-Chihuahua--ELECTROFORESISAtPFN.jpeg|200px]]</center> | [[File:T--Tec-Chihuahua--ELECTROFORESISAtPFN.jpeg|200px]]</center> | ||
| Line 38: | Line 37: | ||
<p align="justify"> | <p align="justify"> | ||
Electrophoresis in a 12% polyacrylamide gel was performed in order to corroborate that the protein of interest was indeed expressed. Each well was loaded with 50 μg of total protein from the soluble extracts of the cell lysates. | Electrophoresis in a 12% polyacrylamide gel was performed in order to corroborate that the protein of interest was indeed expressed. Each well was loaded with 50 μg of total protein from the soluble extracts of the cell lysates. | ||
| − | <center><b>Figure 3.</b> SDS-PAGE (12%) of E. coli BL21 (DE3) transformed with BBa_ K2959010.</center></p> | + | <center>[[File:T--Tec-Chihuahua--Gel.jpeg|500px]]</center> |
| + | <center><b>Figure 3.</b> SDS-PAGE (12%) of <i>E. coli </i>BL21 (DE3) transformed with BBa_ K2959010. (<b>Negative control: </b> <i>E.coli</i> BL21 (DE3) untransformed cells, <b>1. </b>ATPFN1 transformed cells without induction, <b>2. </b>ATFPN1 transformed cells with 0.2mM of IPTG, <b>3. </b>ATFPN1 transformed cells with 0.4mM of IPTG, <b>4. </b>ATFPN1 transformed cells with 1mM of IPTG, <b>5. </b>ATFPN1 transformed cells with 3mM of IPTG, <b>6. </b>ATFPN1 transformed cells with 3mM of IPTG)</center></p> | ||
| + | |||
<p align="justify"> | <p align="justify"> | ||
| − | As shown in | + | As shown in <b>Figure 3</b>, bands are presented at the approximate weight of 14.3 kDa which corresponds to AtPFN1 as corroborated by UniProt. Visible bands of AtPFN1 correspond to protein extracts of transformed <i>E. coli</i> BL21 (DE3) induced with different concentrations of IPTG. No band can be appreciated in the negative control column, following the protein ladder, which belongs to a control of untransformed cells, afirming that the bands are indeed AtPFN1. No basal level expression was observed on the uninduced control (transformed cells with no IPTG, column #1). Therefore, functionality of part BBa_K2959010 as a generator of AtPFN1 under induction by IPTG was confirmed. |
| + | </p> | ||
| + | |||
| + | ===Antifungal Assay=== | ||
| + | <p align="justify"> | ||
| + | <br> | ||
| + | In order to test the antifungal activity of our peptides as well as prove their viability as a mechanism to inhibit <i>Verticillium dahliae</i>, an antifungal susceptibility test on a 96 well plate was carried out by measuring absorbance at 405 nm, wavelength used in standardized protocols to measure growth of filamentous fungi<sup>3</sup>. Due to a lack of time, we couldn’t reach the experimental stage of the project of peptide purification, so experiments were made using soluble protein extracts from our transformed cells’ lysates. Different dilutions of the extracts were prepared which were applied to a spore suspension of <i>V. dahliae</i>. Dilutions of the extracts of untransformed cells were used as controls to prove that inhibition was the result of the peptides and not any other protein contained within the extract. Soluble proteins from <i>E. coli</i> BL21 (DE3) were used as control for the AtPFN1 extract. Concentrations of extracts with peptides were equalized to their controls. Table 1 details the concentrations of each dilution. | ||
| + | </p> | ||
| + | <center><b>Table 1.</b> Protein concentration of soluble protein extract of transformed cells induced to produce AtPFN1 and untransformed BL21 (DE3) control. | ||
| + | <table class="lab-table" style="margin-left: auto; margin-right: auto;"> | ||
| + | <tr> | ||
| + | <th style="background-color: #00806c; color: white;">Dilution</th> | ||
| + | <th style="background-color: #00806c; color: white;">AtPFN1 (mg/mL) </th> | ||
| + | <th style="background-color: #00806c; color: white;">BL21 (DE3) control (mg/mL) </th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>1 (undiluted)</td> | ||
| + | <td> 3.7402 </td> | ||
| + | <td> 3.74</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> 3:4</td> | ||
| + | <td> 2.8052</td> | ||
| + | <td>2.805 </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> 1:2 </td> | ||
| + | <td>1.8701</td> | ||
| + | <td>1.87</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> 1:4</td> | ||
| + | <td>0.9351</td> | ||
| + | <td>0.935</td> | ||
| + | |||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | <p align="justify"> | ||
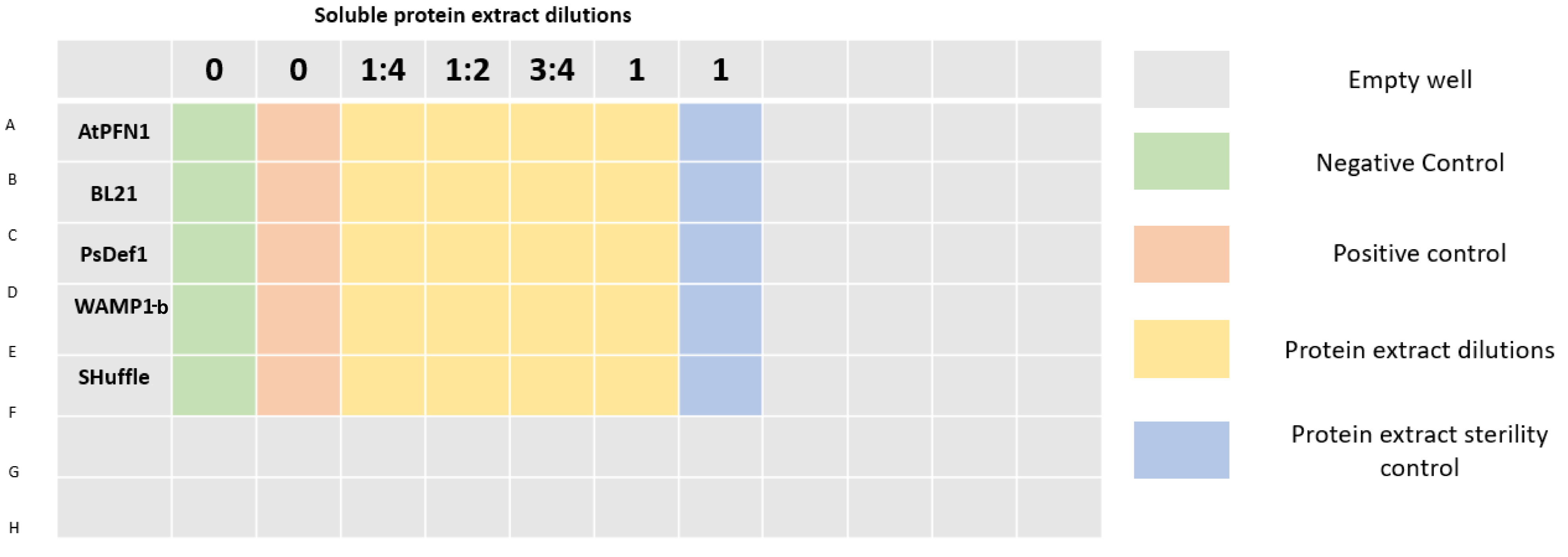
| + | 96 well plates were prepared as shown in <b>Figure 4</b>. A final volume of 200 μL was completed in each well by mixing protein extract, sterile distilled water (to achieve desired concentrations), potato dextrose broth, and a spore suspension of <i>V. dahliae</i> with a final concentration of 2x10<sup>4</sup> spores/mL per well. Plates were incubated at 25°C. | ||
| + | </p> | ||
| + | <br> | ||
| + | [[File:T--Tec-Chihuahua--microFinal.png|800px]] | ||
| + | <p><b>Figure 4.</b> 96 well plate distibution. </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Absorbance readings at 405 nm were performed in a Varioskan Lux 3020-231 microplate reader 1 and 24 hours after plate preparation. After analyzing the results, a growth rate percentage was estimated for every evaluated sample by using the following formula: | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Growth rate = ((A<sub>1</sub> - A<sub>0</sub>)/A<sub>0</sub>) x 100 | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Where:<br> | ||
| + | A<sub>1</sub> = Absorbance after 24 hours.<br> | ||
| + | A<sub>0</sub> = Absorbance after 1 hour. | ||
| + | </p> | ||
| + | |||
| + | ===Results=== | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | Results generated by the previous formula correspond to the percentage in which absorbance increased in each well compared to the initial reading. The results are summarized in <b>Figure 5</b>. As the figure shows, an outstanding difference in growth values exists among the positive growth controls without any kind of protein extract, the untransformed strain extract controls, and the growth of the fungus with different peptide concentrations, was noted. The positive control was estimated to grow at a rate of 40.36% after 24 h, on the other hand, the lowest concentrations of added protein extracts clearly showed a decreasing behavior in growth rates. In comparison, growth rates from wells containing protein extracts of untransformed cells do not behave this way, meaning that AtPFN1 had a degree of inhibition against <i>V. dahliae</i>, even in crude extract. | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | In order to better comprehend the results, growth rates were used to estimate the percentage of inhibition using the following formula: | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | I = 100 - (Gp/Gv) x 100<br> | ||
| + | Where:<br> | ||
| + | I = Inhibition %<br> | ||
| + | Gp = Growth rate of <i>V. dahliae</i> treated with extract with peptides.<br> | ||
| + | Gv = Growth rate of <i>V. dahliae</i> positive control. | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Untransformed <i>E. coli</i> BL21 (DE3) protein extracts seemed to increase the fungus’ growth in contrast with the extract containing the AtPFN1 peptide, which demonstrated inhibition in all three dilutions with the exception of the undiluted extract. The inhibition percentages for this peptide were of 30.40%, 76.96%, 98.32% for dilutions 3:4, 1:2, and 1:4 respectively in comparison with the positive growth control. This assay was able to demonstrate the peptide’s reported ability to permeabilize the cell wall and membrane of fungal spores<sup>2</sup> by showing growth reduction in almost all dilutions and close to total inhibition in the last one, as shown in <b>figure 5</b>.</p> | ||
| + | [[File:T--Tec-Chihuahua--Uno.jpeg|800px]] | ||
| + | <br> | ||
| + | <b>Figure 5.</b> Antifungal activity of AtPFN1 against <i>V. dahliae.</i> <i>V. dahliae</i> growth rate (%) challenged with different concentrations of soluble protein extract from transformed <i>E. coli</i> BL21 (DE3) with AtPFN1 and untransformed <i>E. coli</i> BL21 (DE3). | ||
| + | |||
| + | |||
| + | |||
| + | ===Discussion=== | ||
| + | <br> | ||
| + | <p align="justify"> | ||
| + | When evaluating the results, we looked for an explanation for the inhibitory effect of the peptide occuring at lower concentrations of crude extract. A common occurrence in microplates assays is the precipitation of the inhibitory components, which leads to inconsistent inhibition values.<sup>4</sup> Peptides tend to precipitate at certain concentrations, depending on their composition, solubility and even the buffer or medium they’re diluted in.<sup>4, 5</sup> Given that we have almost no concrete information about our peptides, and that we saw ourselves forced to use crude extract, precipitation at the highest concentrations we used is a feasible possibility. It has been remarked in literature that this incidence makes it almost impossible to determine the MIC (Minimal Inhibitory Concentration) through this method.<sup>4</sup> | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | The precipitation of our peptides could have affected the optic density measurements causing variations that altered the overall results.<sup>5</sup> Or, most likely, the precipitation altered the inhibitory effect of our peptides at high concentrations. This is a common occurrence, and recommendations can be found through literature, research papers, and protocol manuals.<sup>6</sup> | ||
</p> | </p> | ||
| Line 54: | Line 147: | ||
<br> | <br> | ||
2. Park, S. C., Kim, I. R., Kim, J. Y., Lee, Y., Kim, E. J., Jung, J. H., ... & Lee, J. R. (2018). Molecular mechanism of Arabidopsis thaliana profilins as antifungal proteins. Biochimica et Biophysica Acta (BBA)-General Subjects, 1862(12), 2545-2554. doi:10.1016/j.bbagen.2018.07.028 | 2. Park, S. C., Kim, I. R., Kim, J. Y., Lee, Y., Kim, E. J., Jung, J. H., ... & Lee, J. R. (2018). Molecular mechanism of Arabidopsis thaliana profilins as antifungal proteins. Biochimica et Biophysica Acta (BBA)-General Subjects, 1862(12), 2545-2554. doi:10.1016/j.bbagen.2018.07.028 | ||
| + | <br> | ||
| + | 3. Schwalbe, R., Steele-Moore, L., & Goodwin, A. C. (2007). Antimicrobial susceptibility testing protocols. Crc Press. | ||
| + | <br> | ||
| + | 4. Eloff, J. (1998). A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Medica, 64(08), 711–713. doi:10.1055/s-2006-957563 | ||
| + | <br> | ||
| + | 5. Morishige, H., Mano, Y., Oguri, T., & Furuya, N. (2012). Comparison of four reading methods of broth microdilution based on the Clinical and Laboratory Standards Institute M27-A3 method for Candida spp. THE JAPANESE JOURNAL OF ANTIBIOTICS , 65(5), 335–347. Retrieved from http://jja-contents.wdc-jp.com/pdf/JJA65/65-5/65-5_335-347.pdf | ||
| + | <br> | ||
| + | 6. Berditsch, M. (2012). Two-fold Broth Microdilution Method for Determination of MIC . Institute for Bio and Geosciences. Retrieved from http://www.ibg.kit.edu/nmr/downloads/MICprotocoll_30_Jan2012.pdf | ||
<br> | <br> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 03:03, 22 October 2019
Expressible Arabidopsis thaliana Profilin 1
This composite part consists of a T7 promoter, ribosome binding site, a coding sequence for AtPFN1 as a fusion protein with a 6x His-Tag, and a double terminator. This construct allows the expression of AtPFN, an antifungal peptide from Arabidopsis thaliana in E. coli BL21 (DE3). Expression can be positively regulates by the addition of IPTG thanks to its promoter. The part is designed to code for a fusion protein of AtPFN1 with a polyhistidine tag (6x His-Tag) at its C-terminus for purification by immobilized metal affinity chromatography.
Usage and Biology
AtPFN1 is a protein extracted from the plant Arabidopsis thaliana, it is a profillin which means that is an actin binding protein and weights 14 kDa1. It inhibits fungal cells growth by penetrating the cell wall and membrane, generating reactive oxygen species and mitochondrial superoxide triggering cell apoptosis, resulting in morphological changes in the cells2.
The binding affinity of antifungal proteins to fungal cells is the most important attribute for their fungal action, even if the mechanism is membranolytic or cell damaging. It has also been demonstrated that these proteins can be transferred across the cell membrane into the cytosolic space and accumulate in the cytosol of the cell by altering the membrane integrity. For cytosolic translocation the mechanisms used by the proteins are direct penetration, vacuolar localization and expansion, partial plasma membrane disruption, transition pore formation and endocytosis. AtPFN1 has exhibited a potent antifungal activity against fungal strains of C. gloesporioides, F. osysporum, C. albicans, and C. glabrata2.
Characterization of Expressible Arabidopsis thaliana Profilin 1
Our DNA sequence AtPFN1, was synthesized by IDT®️ with the Biobrick prefix and suffix flanking the composite part. This made possible the correct digestion with restriction enzymes EcoRI-HF and PstI. After the digestion, ligation was performed with T7 ligase in order to place our construct into the pSB1C3 linearized backbone with chloramphenicol resistance, which was previously digested with the same restriction enzymes. Using the SnapGene®️ software, we could model our ligated expression plasmid, and the final parts resulted in a sequence of 2,651bp. Thereupon, Escherichia coli BL21(DE3) was transformed by heat shock for following antibiotic selection of clones.

The next step was to amplify our BioBrick sequence through colony PCR performed upon our transformed cells to confirm the presence of our expression plasmid inside of our chassis. With the help of the specific forward Biobrick prefix [BBa_G1004] and the specific reverse Biobrick suffix [BBa_G1005], we were able to amplify our sequence exclusively. Through an agarose gel we confirmed the correct transformation. The PCR action from SnapGene®️ was used to predict the size of the amplified sequence which resulted in a size of 640 bp.


Protein production
IPTG Induction and Extraction
Following the construction of the BioBrick, it was necessary to induce protein production. Since T7 promoter for AtPFN1 is used due its high levels of transcription in E.coli BL21 (DE3), isopropyl β-D-1 thiogalactopyranoside (IPTG) is employed as an inducer for T7 RNA polymerase production. The concentration of IPTG utilized was 0.2 mM, 0.4mM, 1mM and 3mM, followed by incubating the cultures at 37°C and 225 rpm for 5 hours. This was followed by protein extraction by lysis solution to which lysozyme was added, in order to obtain our soluble peptides.
SDS-PAGE
Electrophoresis in a 12% polyacrylamide gel was performed in order to corroborate that the protein of interest was indeed expressed. Each well was loaded with 50 μg of total protein from the soluble extracts of the cell lysates.

As shown in Figure 3, bands are presented at the approximate weight of 14.3 kDa which corresponds to AtPFN1 as corroborated by UniProt. Visible bands of AtPFN1 correspond to protein extracts of transformed E. coli BL21 (DE3) induced with different concentrations of IPTG. No band can be appreciated in the negative control column, following the protein ladder, which belongs to a control of untransformed cells, afirming that the bands are indeed AtPFN1. No basal level expression was observed on the uninduced control (transformed cells with no IPTG, column #1). Therefore, functionality of part BBa_K2959010 as a generator of AtPFN1 under induction by IPTG was confirmed.
Antifungal Assay
In order to test the antifungal activity of our peptides as well as prove their viability as a mechanism to inhibit Verticillium dahliae, an antifungal susceptibility test on a 96 well plate was carried out by measuring absorbance at 405 nm, wavelength used in standardized protocols to measure growth of filamentous fungi3. Due to a lack of time, we couldn’t reach the experimental stage of the project of peptide purification, so experiments were made using soluble protein extracts from our transformed cells’ lysates. Different dilutions of the extracts were prepared which were applied to a spore suspension of V. dahliae. Dilutions of the extracts of untransformed cells were used as controls to prove that inhibition was the result of the peptides and not any other protein contained within the extract. Soluble proteins from E. coli BL21 (DE3) were used as control for the AtPFN1 extract. Concentrations of extracts with peptides were equalized to their controls. Table 1 details the concentrations of each dilution.
| Dilution | AtPFN1 (mg/mL) | BL21 (DE3) control (mg/mL) |
|---|---|---|
| 1 (undiluted) | 3.7402 | 3.74 |
| 3:4 | 2.8052 | 2.805 |
| 1:2 | 1.8701 | 1.87 |
| 1:4 | 0.9351 | 0.935 |
96 well plates were prepared as shown in Figure 4. A final volume of 200 μL was completed in each well by mixing protein extract, sterile distilled water (to achieve desired concentrations), potato dextrose broth, and a spore suspension of V. dahliae with a final concentration of 2x104 spores/mL per well. Plates were incubated at 25°C.
Figure 4. 96 well plate distibution.
Absorbance readings at 405 nm were performed in a Varioskan Lux 3020-231 microplate reader 1 and 24 hours after plate preparation. After analyzing the results, a growth rate percentage was estimated for every evaluated sample by using the following formula:
Growth rate = ((A1 - A0)/A0) x 100
Where:
A1 = Absorbance after 24 hours.
A0 = Absorbance after 1 hour.
Results
Results generated by the previous formula correspond to the percentage in which absorbance increased in each well compared to the initial reading. The results are summarized in Figure 5. As the figure shows, an outstanding difference in growth values exists among the positive growth controls without any kind of protein extract, the untransformed strain extract controls, and the growth of the fungus with different peptide concentrations, was noted. The positive control was estimated to grow at a rate of 40.36% after 24 h, on the other hand, the lowest concentrations of added protein extracts clearly showed a decreasing behavior in growth rates. In comparison, growth rates from wells containing protein extracts of untransformed cells do not behave this way, meaning that AtPFN1 had a degree of inhibition against V. dahliae, even in crude extract.
In order to better comprehend the results, growth rates were used to estimate the percentage of inhibition using the following formula:
I = 100 - (Gp/Gv) x 100
Where:
I = Inhibition %
Gp = Growth rate of V. dahliae treated with extract with peptides.
Gv = Growth rate of V. dahliae positive control.
Untransformed E. coli BL21 (DE3) protein extracts seemed to increase the fungus’ growth in contrast with the extract containing the AtPFN1 peptide, which demonstrated inhibition in all three dilutions with the exception of the undiluted extract. The inhibition percentages for this peptide were of 30.40%, 76.96%, 98.32% for dilutions 3:4, 1:2, and 1:4 respectively in comparison with the positive growth control. This assay was able to demonstrate the peptide’s reported ability to permeabilize the cell wall and membrane of fungal spores2 by showing growth reduction in almost all dilutions and close to total inhibition in the last one, as shown in figure 5.

Figure 5. Antifungal activity of AtPFN1 against V. dahliae. V. dahliae growth rate (%) challenged with different concentrations of soluble protein extract from transformed E. coli BL21 (DE3) with AtPFN1 and untransformed E. coli BL21 (DE3).
Discussion
When evaluating the results, we looked for an explanation for the inhibitory effect of the peptide occuring at lower concentrations of crude extract. A common occurrence in microplates assays is the precipitation of the inhibitory components, which leads to inconsistent inhibition values.4 Peptides tend to precipitate at certain concentrations, depending on their composition, solubility and even the buffer or medium they’re diluted in.4, 5 Given that we have almost no concrete information about our peptides, and that we saw ourselves forced to use crude extract, precipitation at the highest concentrations we used is a feasible possibility. It has been remarked in literature that this incidence makes it almost impossible to determine the MIC (Minimal Inhibitory Concentration) through this method.4
The precipitation of our peptides could have affected the optic density measurements causing variations that altered the overall results.5 Or, most likely, the precipitation altered the inhibitory effect of our peptides at high concentrations. This is a common occurrence, and recommendations can be found through literature, research papers, and protocol manuals.6
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
1. Christensen, H. E. M., Ramachandran, S., Tan, C.-T., Surana, U., Dong, C.-H., & Chua, N.-H. (1996). Arabidopsis profilins are functionally similar to yeast profilins: identification of a vascular bundle-specific profilin and a pollen-specific profilin. The Plant Journal, 10(2), 269–279. doi:10.1046/j.1365-313x.1996.10020269.x
2. Park, S. C., Kim, I. R., Kim, J. Y., Lee, Y., Kim, E. J., Jung, J. H., ... & Lee, J. R. (2018). Molecular mechanism of Arabidopsis thaliana profilins as antifungal proteins. Biochimica et Biophysica Acta (BBA)-General Subjects, 1862(12), 2545-2554. doi:10.1016/j.bbagen.2018.07.028
3. Schwalbe, R., Steele-Moore, L., & Goodwin, A. C. (2007). Antimicrobial susceptibility testing protocols. Crc Press.
4. Eloff, J. (1998). A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Medica, 64(08), 711–713. doi:10.1055/s-2006-957563
5. Morishige, H., Mano, Y., Oguri, T., & Furuya, N. (2012). Comparison of four reading methods of broth microdilution based on the Clinical and Laboratory Standards Institute M27-A3 method for Candida spp. THE JAPANESE JOURNAL OF ANTIBIOTICS , 65(5), 335–347. Retrieved from http://jja-contents.wdc-jp.com/pdf/JJA65/65-5/65-5_335-347.pdf
6. Berditsch, M. (2012). Two-fold Broth Microdilution Method for Determination of MIC . Institute for Bio and Geosciences. Retrieved from http://www.ibg.kit.edu/nmr/downloads/MICprotocoll_30_Jan2012.pdf