Difference between revisions of "Part:BBa K2912015"
Mackintosh (Talk | contribs) |
Mackintosh (Talk | contribs) (→SZU-China 2019 iGEM team) |
||
| (25 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
<partinfo>BBa_K2912015 short</partinfo> | <partinfo>BBa_K2912015 short</partinfo> | ||
| − | + | Trp_Lysis gene expression can be controlled by the concentration of Tryptophan | |
<!-- --> | <!-- --> | ||
| Line 8: | Line 8: | ||
<partinfo>BBa_K2912015 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2912015 SequenceAndFeatures</partinfo> | ||
| + | ===SZU-China 2019 iGEM team=== | ||
| + | The lysis gene from colicin-producing strains of bacteria encodes the lysis protein which is only 4872Da in molecular weight and performs two functions that are instrumental to the activity of Colicin E7 (ColE7):one of its function is to Release of ColE7 from ColE7—Immunity complex and the other is Partial lysis of host cell membrane, which means that once the protein is produced and activated, the protein can cause the host cell to lyse. | ||
| − | + | On the basis of its special biological function, it's likely to use lysis protein as a cellular structure breaker. In this year, our team decided to add a Tryptophan attenuator between a RBS and the lysis gene, making it possible to switch off or switch on the lysing mechanism by controlling the tryptophan concentration of medium, so that the lysis protein can better serve in our project when we need to break our engineering cells to extract our product. | |
| − | + | ||
| − | + | ||
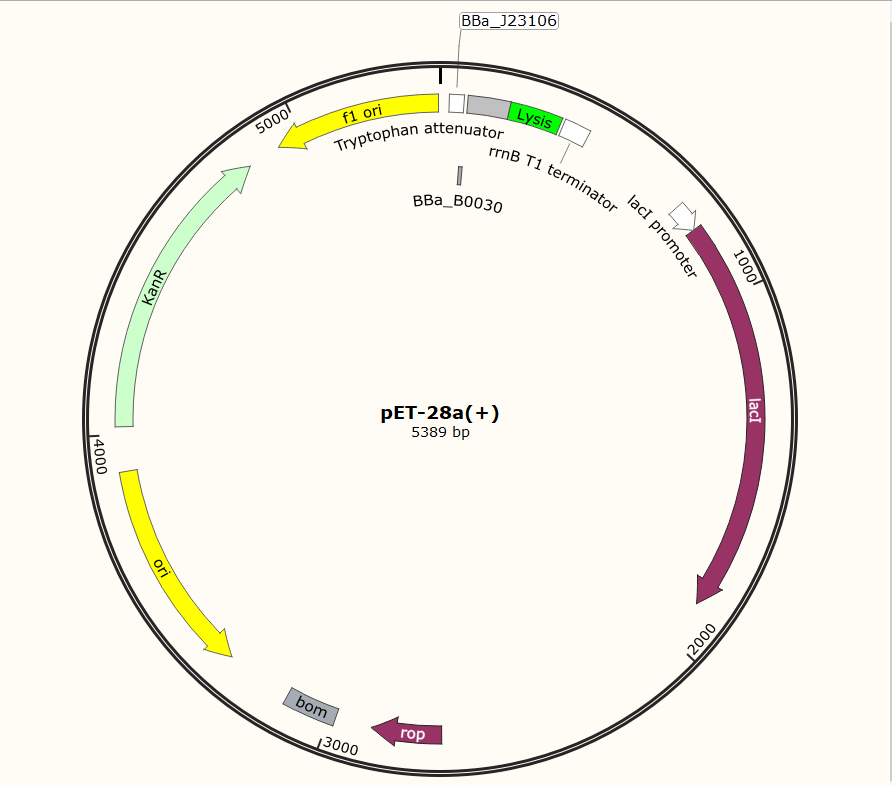
| − | + | SZU-China 2019 iGEM team constructed a plasmid with BBa_K1475900 (Promoter), BBa_K2912014 (Attenuator), BBa_K117000(lysis), BBa_K3257020(terminator)(illustrated in Fig.1). | |
| − | According to the characterization experiment's result, we can draw a conclusion that once the lysis protein is produced | + | <div> |
| + | <center><html><img src='https://2019.igem.org/wiki/images/5/5c/T--SZU-CHINA--fig.1.png' style="width:50%;margin:0 auto"> | ||
| + | <center> Fig.1 Plasmid construction of Trp_lysis </center></html></center> | ||
| + | </div> | ||
| + | |||
| + | Before the official experiment of Trp_Lysis gene. We have done the T7 promotor_lysis gene characterization experiment with IPTG inducing in LB liquid medium(illustrated in Fig.2). | ||
| + | |||
| + | <div> | ||
| + | <center><html><img src='https://2019.igem.org/wiki/images/d/d9/T--SZU-CHINA--fig._7.png' style="width:50%;margin:0 auto"> | ||
| + | <center> Fig.2 OD600 of two different treated T7 promotor_lysis samples </center></html></center> | ||
| + | </div> | ||
| + | |||
| + | According to the characterization experiment's result, we can draw a conclusion that once the lysis protein is produced with IPTG inducing by HT115 (DE3) E.coli, their growth will be significantly inhibited. | ||
| − | In our next step, we transformed the expression vector into HT115 (DE3) E.coli by heat-shock method, culturing in LB liquid medium to exact exponential phase | + | In our next step, we transformed the expression vector into HT115 (DE3) E.coli by heat-shock method, culturing in LB liquid medium to exact exponential phase, and then moved to fresh LB liquid medium with different concentration of tryptophan, starting a eight-hour cell growth curve experiment of our engineering E.coli and then we measured the OD600 of each groups every two hours. Finally, we calculated their survival rate by using OD600 of the experimental group divided by OD600 of the blank group (illustrated in Fig.3). |
| + | |||
| + | <div> | ||
| + | <center><html><img src='https://static.igem.org/mediawiki/parts/3/37/T--SZU-CHINA--improved_new_data.jpg' style="width:50%;margin:0 auto"> | ||
| + | <center> Fig.3 The survival rate of E. coli with Trp-Lysis </center></html></center> | ||
| + | </div> | ||
| + | |||
| + | Besides, we also did a culturing in LB solid medium experiment in tryptophan concentration gradient.(illustrated in Fig.4) | ||
| − | + | <div> | |
| + | <center><html><img src='https://2019.igem.org/wiki/images/4/4e/T--SZU-CHINA--fig._4.png' style="width:50%;margin:0 auto"> | ||
| + | <center> Fig.4 growth of Trp_lysis E.coli under different treated </center></html></center> | ||
| + | </div> | ||
| − | |||
| − | + | <div> | |
| + | <center><html><img src='https://2019.igem.org/wiki/images/6/61/T--SZU-CHINA--fig_5.jpg' style="width:50%;margin:0 auto"> | ||
| + | <center> Fig.5 growth of T7 promotor_lysis E.coli under different treated </center></html></center> | ||
| + | </div> | ||
| + | In contrast with T7 promotor_lysis with IPTG inducing group(illustrated in Fig.5). The Trp_Lysis metabolism obviously has the advantage which is able to kill the host cell by breaking their structure in a controlled way. In other words, we can switch off or switch on the lysing mechanism by controlling the tryptophan concentration of the culture medium, and the result indicates that the concentration critical valve of tryptophan is around 0.3% which means that if the concentration is more than 0.3%, the killing metabolism will be closed so that the host cells can grow as usual. | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 02:56, 22 October 2019
Trp_Lysis gene
Trp_Lysis gene expression can be controlled by the concentration of Tryptophan
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
SZU-China 2019 iGEM team
The lysis gene from colicin-producing strains of bacteria encodes the lysis protein which is only 4872Da in molecular weight and performs two functions that are instrumental to the activity of Colicin E7 (ColE7):one of its function is to Release of ColE7 from ColE7—Immunity complex and the other is Partial lysis of host cell membrane, which means that once the protein is produced and activated, the protein can cause the host cell to lyse.
On the basis of its special biological function, it's likely to use lysis protein as a cellular structure breaker. In this year, our team decided to add a Tryptophan attenuator between a RBS and the lysis gene, making it possible to switch off or switch on the lysing mechanism by controlling the tryptophan concentration of medium, so that the lysis protein can better serve in our project when we need to break our engineering cells to extract our product.
SZU-China 2019 iGEM team constructed a plasmid with BBa_K1475900 (Promoter), BBa_K2912014 (Attenuator), BBa_K117000(lysis), BBa_K3257020(terminator)(illustrated in Fig.1).
Before the official experiment of Trp_Lysis gene. We have done the T7 promotor_lysis gene characterization experiment with IPTG inducing in LB liquid medium(illustrated in Fig.2).
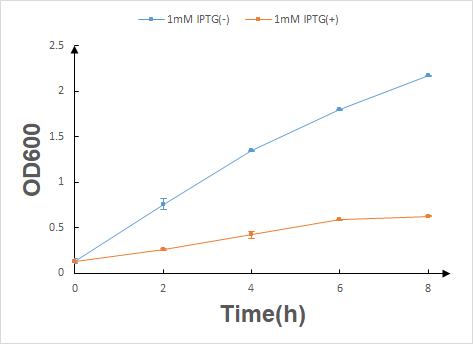
According to the characterization experiment's result, we can draw a conclusion that once the lysis protein is produced with IPTG inducing by HT115 (DE3) E.coli, their growth will be significantly inhibited.
In our next step, we transformed the expression vector into HT115 (DE3) E.coli by heat-shock method, culturing in LB liquid medium to exact exponential phase, and then moved to fresh LB liquid medium with different concentration of tryptophan, starting a eight-hour cell growth curve experiment of our engineering E.coli and then we measured the OD600 of each groups every two hours. Finally, we calculated their survival rate by using OD600 of the experimental group divided by OD600 of the blank group (illustrated in Fig.3).

Besides, we also did a culturing in LB solid medium experiment in tryptophan concentration gradient.(illustrated in Fig.4)
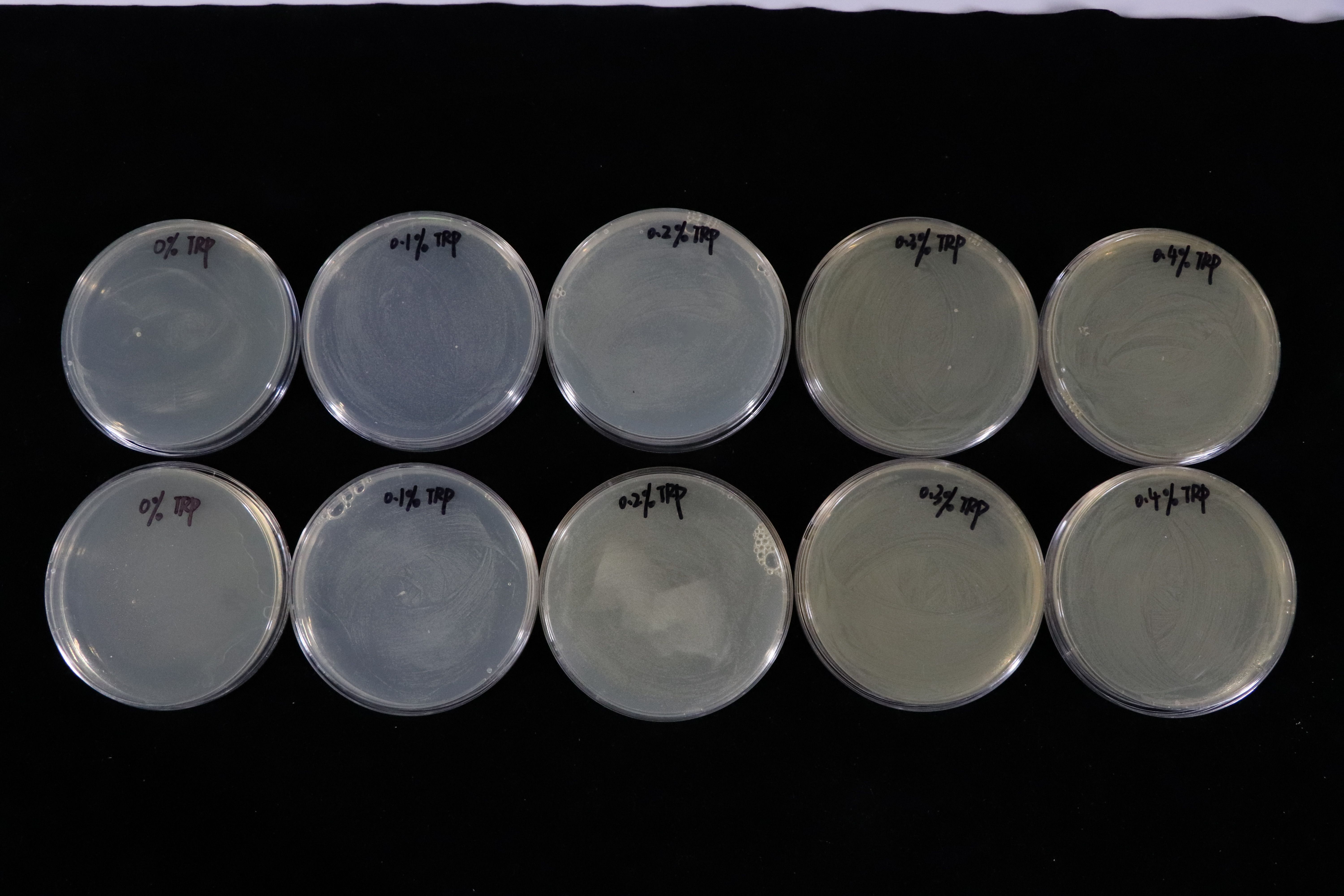
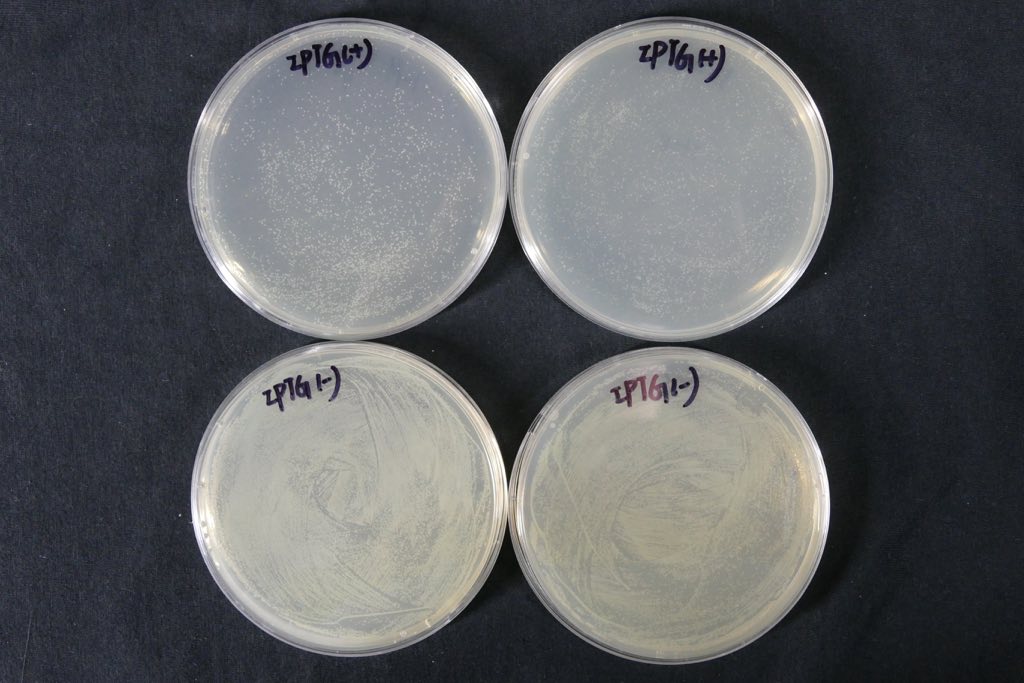
In contrast with T7 promotor_lysis with IPTG inducing group(illustrated in Fig.5). The Trp_Lysis metabolism obviously has the advantage which is able to kill the host cell by breaking their structure in a controlled way. In other words, we can switch off or switch on the lysing mechanism by controlling the tryptophan concentration of the culture medium, and the result indicates that the concentration critical valve of tryptophan is around 0.3% which means that if the concentration is more than 0.3%, the killing metabolism will be closed so that the host cells can grow as usual.
