Difference between revisions of "Part:BBa K3264005"
(→Reference) |
|||
| Line 40: | Line 40: | ||
==Reference== | ==Reference== | ||
[1]Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269. | [1]Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269. | ||
| + | |||
[2]Zhou, Yizhong, et al. “Production and Properties of Triple Chimeric Spidroins.” Biomacromolecules, vol. 19, no. 7, 2018, pp. 2825–2833., doi:10.1021/acs.biomac.8b00402. | [2]Zhou, Yizhong, et al. “Production and Properties of Triple Chimeric Spidroins.” Biomacromolecules, vol. 19, no. 7, 2018, pp. 2825–2833., doi:10.1021/acs.biomac.8b00402. | ||
Revision as of 22:21, 21 October 2019
NT4RepCT
NT-4Rep-CT is the recombinant spider silk protein (spidroin) that can spin into artificial spider silk fibers. This part is in the part collection where we provide improved recombinant chromoproteins and spidroins based on the functions of different regions of naturally occurring spidroin. By carrying out different mixing of the proteins of different combinations, we can produce artificially spun silk of different colors and properties.
The part collection includes: Parts that are different kinds of spidroins for silk spining. BBa_K3264000 BBa_K3264005 BBa_K3264007 BBa_K3264009 BBa_K3264018. BBa_K3264019. Parts that for combining with the spidroins to form color silks BBa_K3264012. BBa_K3264013. BBa_K3264014. BBa_K3264015. BBa_K3264016. BBa_K3264017. Part for adding more extensive repetitive region to the spidroin protein BBa_K3264011.
Our part collection can help and inspire future teams to further design recombinant spider silks of more variety and give silks diversified color and property to fulfill the collection.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal SpeI site found at 943
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 424
Illegal SpeI site found at 943 - 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal SpeI site found at 943
- 25INCOMPATIBLE WITH RFC[25]Illegal SpeI site found at 943
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Spider silk serves as a new material with superior properties that can be applied in medication, cloth, and aerospace fields. However, spider breeding is not applicable due to the spider's fierce behavior. The current approach is to produce recombinant spidroins (silk proteins) from other chassis and spin them into silk. Enable a step closer to unobtainable size (556 kDa) of natural spider spidroin, containing 192 repeat motifs of the Nephila clavipes dragline spidroin, we have developed a synthetic biology approach where we combine standardized DNA part assembly and split the intermediate ligation to produce recombinant spidroins of NT-4Rep-CT, in this case, extra a 2Rep is added to the NT-2Rep-CT and was expected to have a better mechanical performance after spinning. NT-2Rep-CT is the common architecture in two main types of spider silk proteins: major ambulate spidorins(MaSps) and minor ampullate spidorins (MiSps). NT is a non-repetitive N-terminal domain, as well as CT, the C-terminal domain. In between them is an extensive repetitive region-Rep In the storage of spider silk protein, NT is a monomeric and highly soluble region in neutral pH, and it provides the solubility of the entire spidroin. NT can form stable dimers when pH decreases in the spinning duct. On the other side during the decrease of pH, the CT gets disrupted and unfold to turn in the beta-sheet amyloid-like fibrils. It is hypothesized that structural change of CT precipitates the change of the repetitive region to beta-sheet conformation.
Reference
[1]Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269.
[2]Zhou, Yizhong, et al. “Production and Properties of Triple Chimeric Spidroins.” Biomacromolecules, vol. 19, no. 7, 2018, pp. 2825–2833., doi:10.1021/acs.biomac.8b00402.
Characterization
We aimed to use a synthetic biology approach to combine standardized DNA part NT-4Rep-CT, and transform constructs to metabolically engineered E. coli for bioproduction.
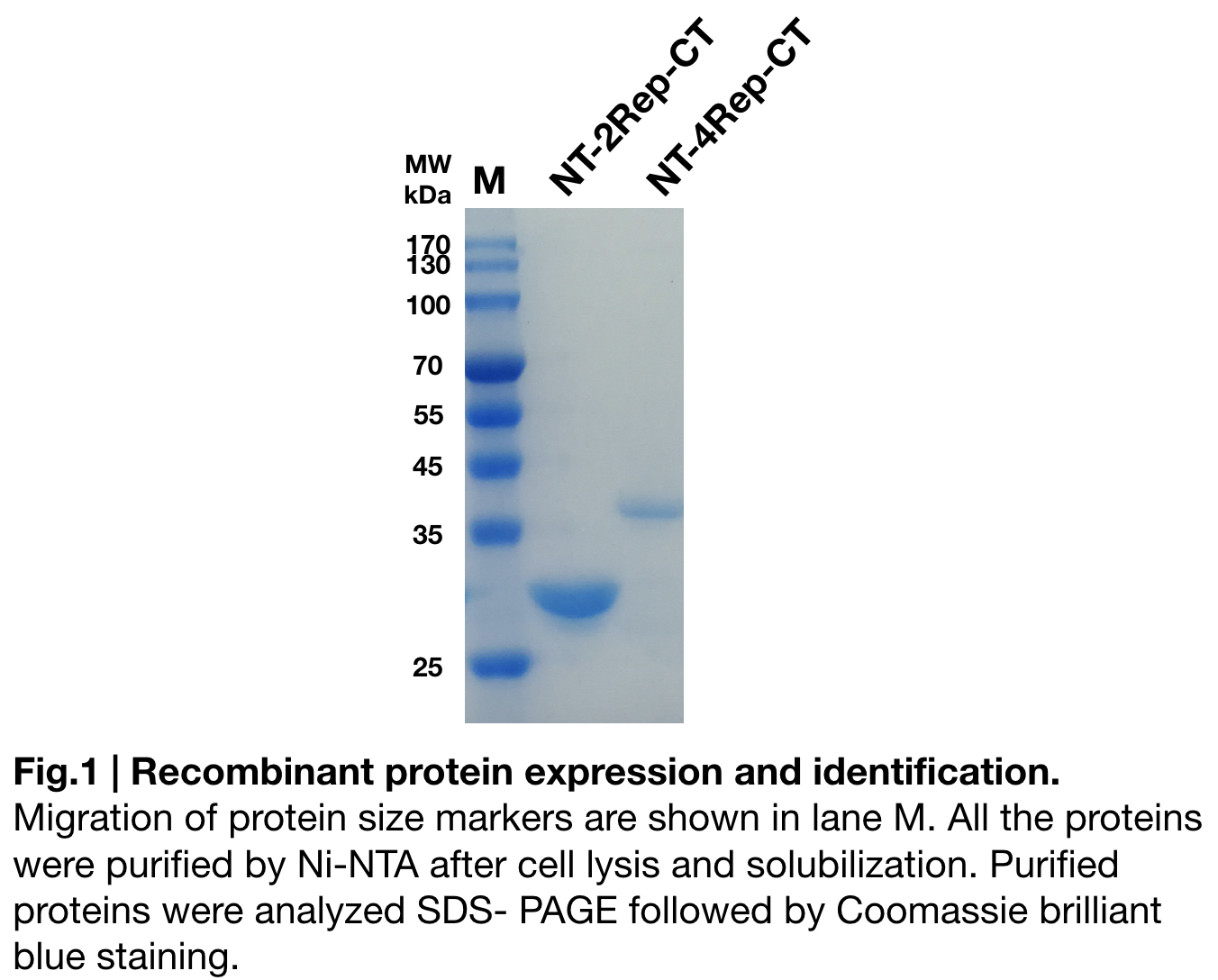
Purification and SDS PAGE
In order to detect whether NT-4Rep-CT protein expression was induced by adding isopropylthiogalactoside (final concentration 0.3mM), we used SDS-page(12%) to determine the presence of target protein. Target protein, NT-4Rep-CT was successfully induced and resulted in a higher molecular weight as it shown on the SDS-page than NT-2Rep-CT (Fig.1). The increase in molecular weight is regarded as reasonable because of the 2Rep added to the original NT-2Rep-CT.