Difference between revisions of "Part:BBa K3171175"
| (7 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3171175 short</partinfo> | <partinfo>BBa_K3171175 short</partinfo> | ||
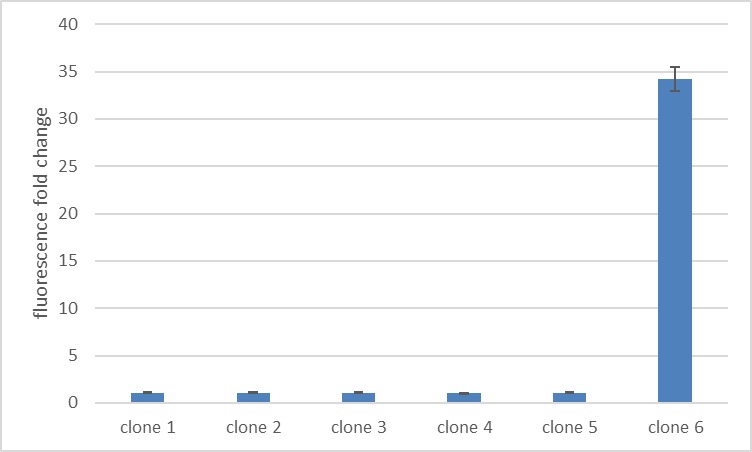
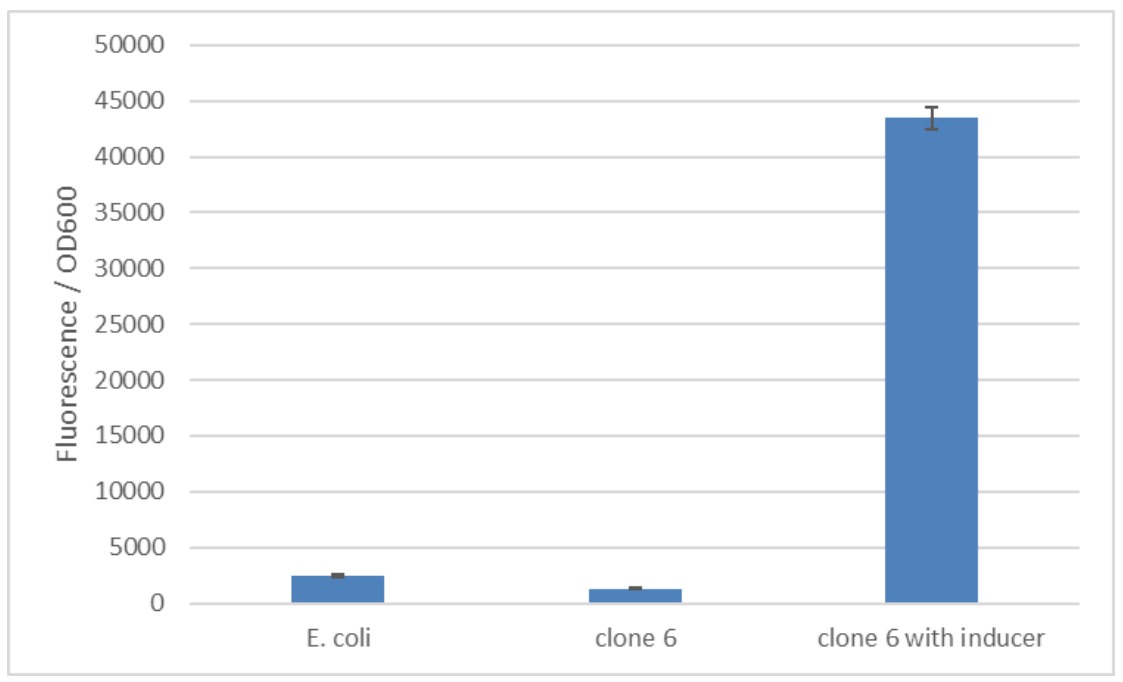
| − | + | We have improved the pTET promoter's function and inducibility for use in <i>E. coli</i> employing a synthetic promoter library on pTet [[Part:BBa_R0040]]. The SPL was constructed using a PCR with randomized primers to yield arbitrary sequences in the promoter region. A total of 245 clones were screened to find a target with enhanced functionality. For the screening the cells were induced with 250 ng/mL anhydrotetracycline. The expression of mCherry was quantified based on the emitted fluorescence levels 8 hours after induction. The screening of the final 6 interesting variations revealed clone 6 as the sequence with increased inducibility (figure 1). The best version of the promoter was determined based on fold change in the fluorescence intensity and low basal level. We calculated a fold change of 34 comparing fluorescence in the induced and uninduced state! Additionally, this promoter also exhibited a low basal level of fluorescence that is comparable to background fluorescence of <i>E. coli</i> without any reporter (figure 2). The improved pTET promoter can be found at [[Part:BBa_K3171173]]. | |
| + | |||
| + | [[File:pTet.jpeg|500px|]] | ||
| + | |||
| + | Figure 1: Clone 6 exhibits the highest fluorescence fold change upon induction. Single clones from the synthetic promoter library were induced with 250 ng/ml anhydrotetracycline. The fluorescence fold change is calculated by dividing the fluorescence in the induced state by fluorescence in the absence of inducer. The used fluorescence reporter is mCherry. | ||
| + | |||
| + | [[File:BBa_K3171173 (1).png|500px|]] | ||
| + | |||
| + | Figure 2: The basal level of reporter expression is comparable to <i>E. coli</i> without the construct. Clone 6 from the synthetic promoter library was induced with 250 ng/ml anhydrotetracycline. The used fluorescence reporter is mCherry. | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | |||
<!-- --> | <!-- --> | ||
Latest revision as of 22:01, 21 October 2019
pTet-mCherry optimized in E. coli
We have improved the pTET promoter's function and inducibility for use in E. coli employing a synthetic promoter library on pTet Part:BBa_R0040. The SPL was constructed using a PCR with randomized primers to yield arbitrary sequences in the promoter region. A total of 245 clones were screened to find a target with enhanced functionality. For the screening the cells were induced with 250 ng/mL anhydrotetracycline. The expression of mCherry was quantified based on the emitted fluorescence levels 8 hours after induction. The screening of the final 6 interesting variations revealed clone 6 as the sequence with increased inducibility (figure 1). The best version of the promoter was determined based on fold change in the fluorescence intensity and low basal level. We calculated a fold change of 34 comparing fluorescence in the induced and uninduced state! Additionally, this promoter also exhibited a low basal level of fluorescence that is comparable to background fluorescence of E. coli without any reporter (figure 2). The improved pTET promoter can be found at Part:BBa_K3171173.
Figure 1: Clone 6 exhibits the highest fluorescence fold change upon induction. Single clones from the synthetic promoter library were induced with 250 ng/ml anhydrotetracycline. The fluorescence fold change is calculated by dividing the fluorescence in the induced state by fluorescence in the absence of inducer. The used fluorescence reporter is mCherry.
Figure 2: The basal level of reporter expression is comparable to E. coli without the construct. Clone 6 from the synthetic promoter library was induced with 250 ng/ml anhydrotetracycline. The used fluorescence reporter is mCherry.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]