Difference between revisions of "Part:BBa J23115"
| (11 intermediate revisions by 5 users not shown) | |||
| Line 12: | Line 12: | ||
==Lambert_GA 2019 Characterization== | ==Lambert_GA 2019 Characterization== | ||
Lambert_GA 2019 tested several combinations of constitutive promoters and ribosomal binding sites to characterize each by measuring enzyme activity and therefore protein expression. The gene expressed, LacZ, codes for β-galactosidase (β-gal), which typically breaks down lactose. Instead of using lactose, we added the sugar ONPG (Ortho-Nitrophenyl-β-galactoside). β-gal breaks ONPG down into galactose and ONP (Ortho-Nitrophenol), which has a yellow color. If there is more ONP present, there is more enzymatic activity and therefore more expression of LacZ. We used a plate reader to measure absorbance at 420 nm, measuring yellow color, and 600nm, measuring cell density. We inputted those absorbance values into the Miller unit formula to calculate enzymatic activity per cell per milliliter. | Lambert_GA 2019 tested several combinations of constitutive promoters and ribosomal binding sites to characterize each by measuring enzyme activity and therefore protein expression. The gene expressed, LacZ, codes for β-galactosidase (β-gal), which typically breaks down lactose. Instead of using lactose, we added the sugar ONPG (Ortho-Nitrophenyl-β-galactoside). β-gal breaks ONPG down into galactose and ONP (Ortho-Nitrophenol), which has a yellow color. If there is more ONP present, there is more enzymatic activity and therefore more expression of LacZ. We used a plate reader to measure absorbance at 420 nm, measuring yellow color, and 600nm, measuring cell density. We inputted those absorbance values into the Miller unit formula to calculate enzymatic activity per cell per milliliter. | ||
| + | <br> | ||
| + | <br> | ||
| + | <html> | ||
| + | <style> | ||
| + | table, th, td { | ||
| + | border: 1px solid black; | ||
| + | border-collapse: collapse; | ||
| + | } | ||
| + | th, td { | ||
| + | padding: 15px; | ||
| + | } | ||
| + | </style> | ||
| + | </head> | ||
| + | <body> | ||
| + | |||
| + | <table style="width:100%"> | ||
| + | <tr> | ||
| + | <th>Strain Identification Number </th> | ||
| + | <th>Promoter Part Number</th> | ||
| + | <th>RBS Part Number</th> | ||
| + | <th>Relative Strength of Promoter/RBS</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>R (positive control)</td> | ||
| + | <td>BBa_J23115</td> | ||
| + | <td>BBa_B0035</td> | ||
| + | <td>Reference/Reference</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>1</td> | ||
| + | <td>BBa_J23113</td> | ||
| + | <td>BBa_B0031</td> | ||
| + | <td>Weak/Weak</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>2</td> | ||
| + | <td>BBa_J23113</td> | ||
| + | <td>BBa_B0032</td> | ||
| + | <td>Weak/Medium</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>3</td> | ||
| + | <td>BBa_J23113</td> | ||
| + | <td>BBa_B0034</td> | ||
| + | <td>Weak/Strong</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>4</td> | ||
| + | <td>BBa_J23106</td> | ||
| + | <td>BBa_B0031</td> | ||
| + | <td>Medium/Weak</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>5</td> | ||
| + | <td>BBa_J23106</td> | ||
| + | <td>BBa_B0032</td> | ||
| + | <td>Medium/Medium</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>6</td> | ||
| + | <td>BBa_J23106</td> | ||
| + | <td>BBa_B0034</td> | ||
| + | <td>Medium/Strong</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>7</td> | ||
| + | <td>BBa_J23119</td> | ||
| + | <td>BBa_B0031</td> | ||
| + | <td>Strong/Weak</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>8</td> | ||
| + | <td>BBa_J23119</td> | ||
| + | <td>BBa_B0032</td> | ||
| + | <td>Strong/Medium</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>9</td> | ||
| + | <td>BBa_J23119</td> | ||
| + | <td>BBa_B0034</td> | ||
| + | <td>Strong/Strong</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </body> | ||
| + | </html> | ||
| + | <br> | ||
| + | <center> [[File:T--Lambert GA--tuningreference.png|800px]] | ||
| + | <br> | ||
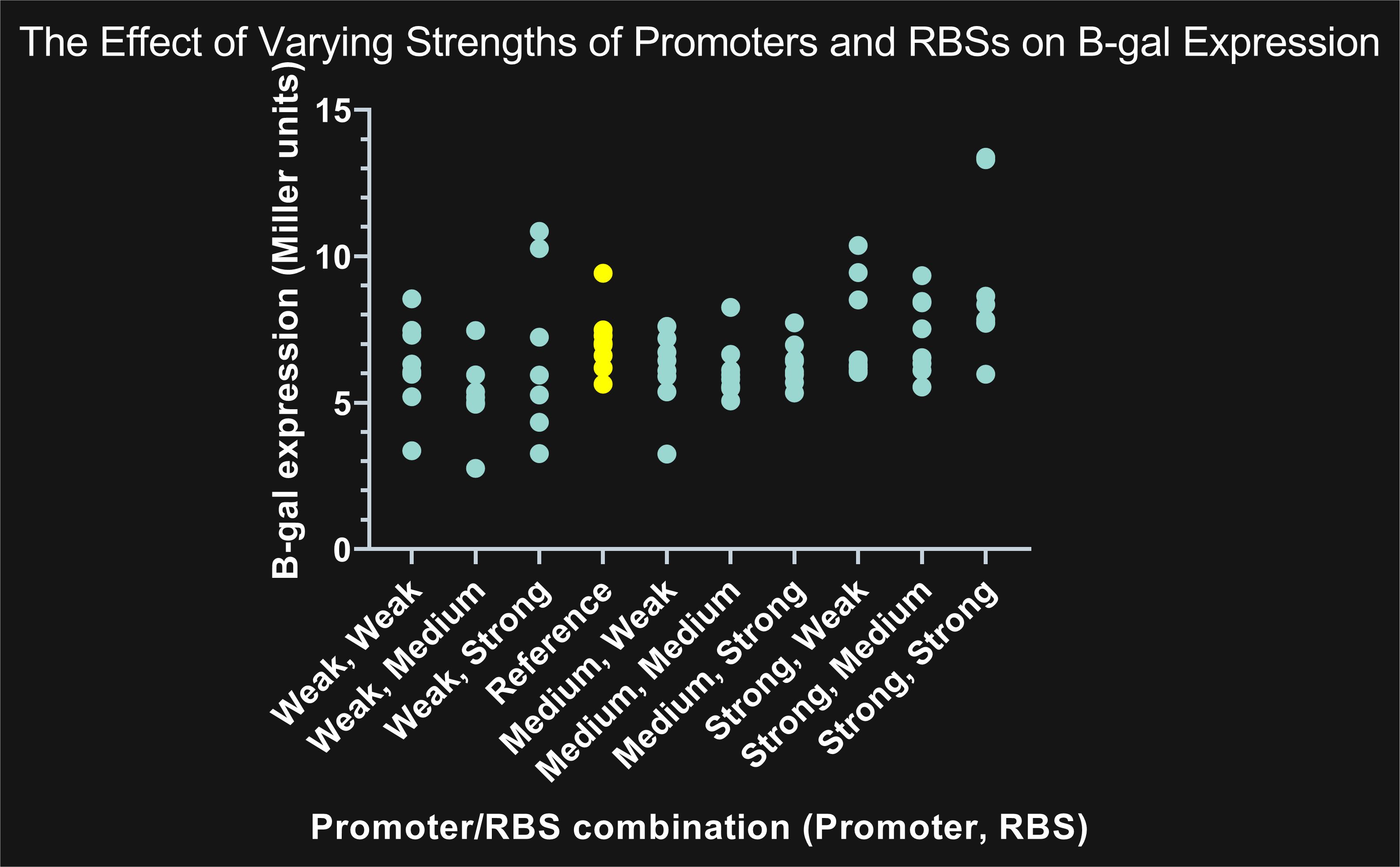
| + | <i>Figure 1: The highlighted points in yellow represent the combination of BBa_J23115 (reference promoter) with BBa_B0035 (reference RBS) compared to the remaining nine combinations highlighted in blue. Combinations showing promoters with the same strength, but different RBS, share similar expression. An increase in promoter strength results in an increase in expression; on the other hand, changes in RBS strength have a negligible effect on expression.</i></center> | ||
| + | <br> | ||
| + | <center>After we calculated Miller units, the data showed the reference’s level of expression was between the weak and medium promoter’s. As a result, the reference was graphed between the weak and medium promoter.</center> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| Line 20: | Line 111: | ||
==USTC_2009's MEASUREMENT== | ==USTC_2009's MEASUREMENT== | ||
[https://parts.igem.org/Part:BBa_K176014 K176014] | [https://parts.igem.org/Part:BBa_K176014 K176014] | ||
| + | |||
| + | |||
| + | |||
| + | {|width='100%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_J23100 AddReview 5</partinfo> | ||
| + | <I>University of Texas at Austin iGEM 2019</I> | ||
| + | |width='60%' valign='top'| | ||
| + | |||
| + | <h3>UT Austin iGEM 2019: Characterization of metabolic burden of the Anderson Series</h3> | ||
| + | |||
| + | <h4>Description</h4> | ||
| + | The 2019 UT Austin iGEM team transformed the Anderson Series promoters into our 'burden monitor' DH10B strain of E. coli, which contains a constitutive GFP cassette in the genome of the cell. GFP expression fluctuates depending on the number of ribosomes available. Using this strain, we characterized the relative burden (percent reduction in growth rate) of each Anderson Series part. Our results showed a range of growth rate reductions for each of these parts due to ribosomal reallocation from the genome of the host cell, towards the expression of RFP. Anderson Series parts with strong promoters are depicted with darker red colors and Anderson Series parts with weak promoters are depicted with lighter pink colors to show relative RFP expression. | ||
| + | We saw a positive correlation between relative promoter strength and metabolic burden; parts with stronger promoters expressed less GFP and had a lower growth rate than parts with weaker promoters. The regression line for the graph below was constructed by measuring the burden of 5 parts that were created by the 2019 UT Austin iGEM team that each contained an Anderson Series promoter (<partinfo>J23104</partinfo> or <partinfo>J23110</partinfo>), an RBS of varying strength, and a BFP reporter. For more information on characterization of these parts through the burden monitor, visit our team’s wiki page: [https://https://2019.igem.org/Team:Austin_UTexas] | ||
| + | |||
| + | <html> | ||
| + | <figure> | ||
| + | <div class = "left"> | ||
| + | <img src = "https://static.igem.org/mediawiki/parts/a/a0/AndersonCharacterization.jpg" style = "width:550px;height:500px"> | ||
| + | </div> | ||
| + | <figcaption><b>Fig.1:</b>Growth vs GFP Expression graph showing the relative burden positions of the Anderson Series promoters. The parts with strong promoters are depicted in dark red and are clustered near the bottom of the graph because they have lower growth rates and express lower levels of GFP as a result of high cellular burden. The parts with weaker promoter are depicted in light pink ad are clustered near the top of the graph because they have higher growth rates and express higher levels of GFP as a result of low cellular burden.</figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | <br><br> | ||
| + | <html> | ||
| + | <figure> | ||
| + | <div class = "left"> | ||
| + | <img src = "https://static.igem.org/mediawiki/parts/8/80/T--Austin_Utexas--andersontable.png" style = "width:545px;height:375px"> | ||
| + | </div> | ||
| + | <figcaption><b>Table.1:</b> Burden measurements for the Anderson Series promoters measured as percent reduction in growth rate ± 95% confidence interval. </figcaption> | ||
| + | </figure> | ||
| + | </html> | ||
| + | |||
| + | <h4>Importance of Characterizing Burden</h4> | ||
| + | <p> Although often we cannot avoid using a specific burdensome part, knowing in advance that it is burdensome, and that it has a high chance of mutating into a non-functional genetic device, can help with troubleshooting and coming up with alternatives. In the specific case of fluorescent protein-expressing devices, Fluorescence-activated cell sorting (FACS) can be used to filter out individual cells that meet a certain fluorescence threshold. This way, the cells expressing lower levels of the fluorescent protein are weeded out of the population.</p> | ||
Latest revision as of 20:18, 21 October 2019
constitutive promoter family member
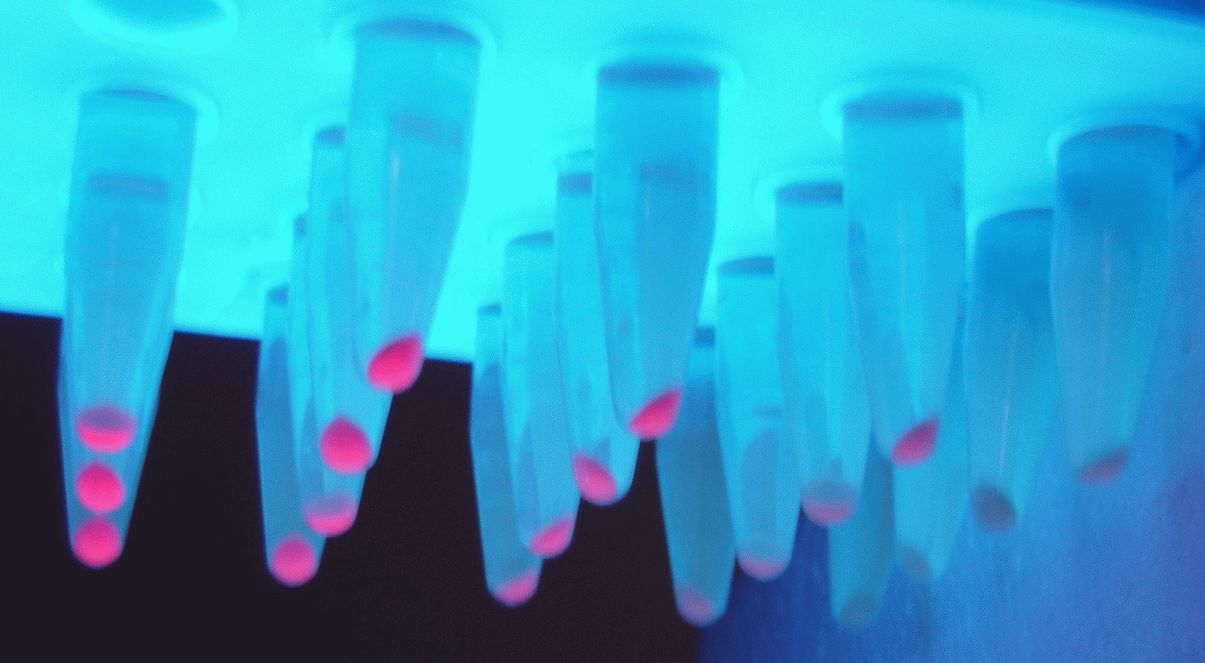
Variant RFP (au) J23112 1 J23103 17 J23113 21 J23109 106 J23117 162 J23114 256 J23115 387 J23116 396 J23105 623 J23110 844 J23107 908 J23106 1185 J23108 1303 J23118 1429 J23111 1487 J23101 1791 J23104 1831 J23102 2179 J23100 2547 |
Constitutive promoter family
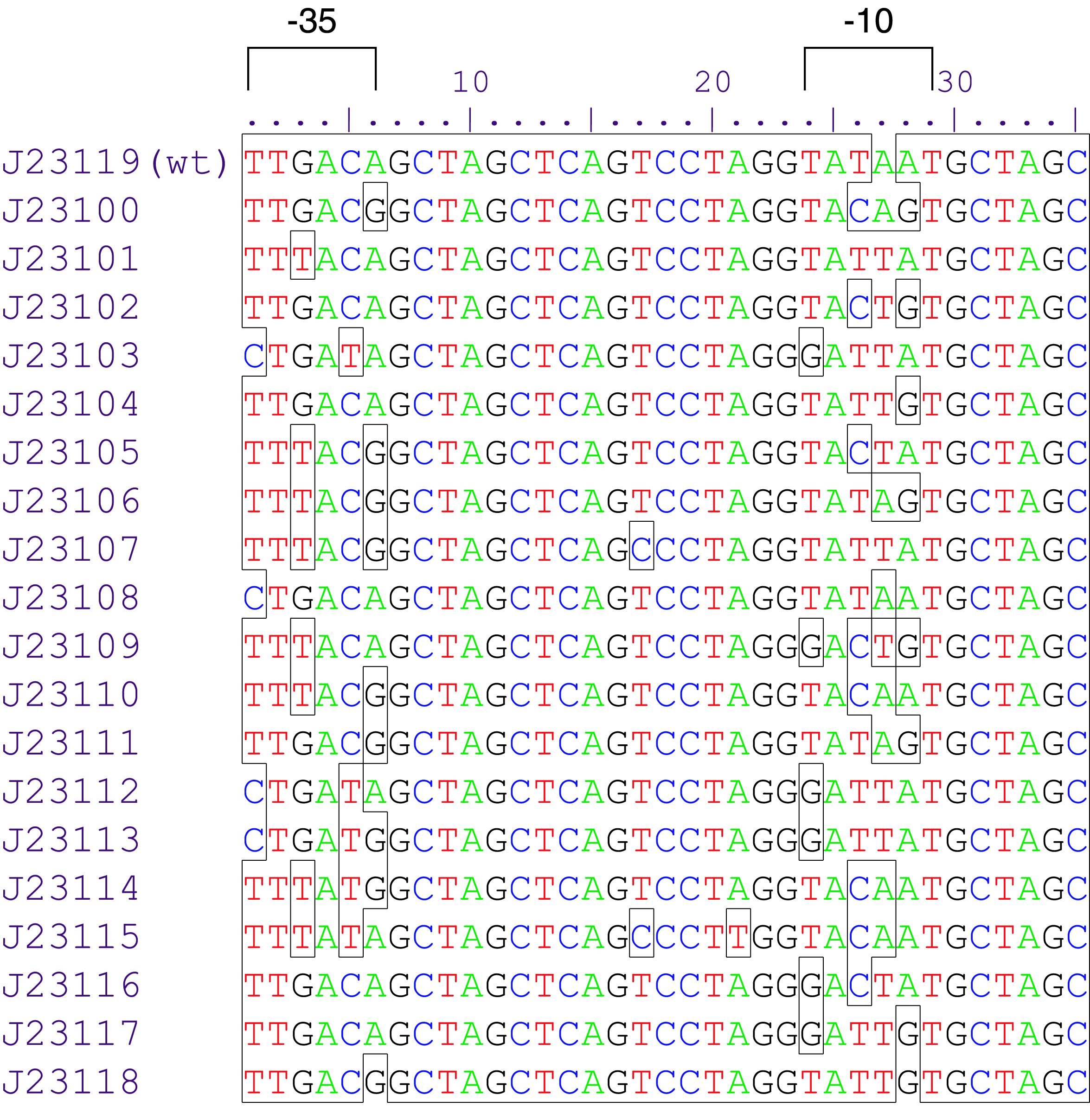
Parts J23100 through J23119 are a family of constitutive promoter parts isolated from a small combinatorial library. J23119 is the "consensus" promoter sequence and the strongest member of the family. All parts except J23119 are present in plasmid J61002. Part J23119 is present in pSB1A2. This places the RFP downstream of the promoter. Reported activities of the promoters are given as the relative fluorescence of these plasmids in strain TG1 grown in LB media to saturation. See part BBa_J61002 for details on their use.
These promoter parts can be used to tune the expression level of constitutively expressed parts. The NheI and AvrII restriction sites present within these promoter parts make them a scaffold for further modification. JCAraw
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Lambert_GA 2019 Characterization
Lambert_GA 2019 tested several combinations of constitutive promoters and ribosomal binding sites to characterize each by measuring enzyme activity and therefore protein expression. The gene expressed, LacZ, codes for β-galactosidase (β-gal), which typically breaks down lactose. Instead of using lactose, we added the sugar ONPG (Ortho-Nitrophenyl-β-galactoside). β-gal breaks ONPG down into galactose and ONP (Ortho-Nitrophenol), which has a yellow color. If there is more ONP present, there is more enzymatic activity and therefore more expression of LacZ. We used a plate reader to measure absorbance at 420 nm, measuring yellow color, and 600nm, measuring cell density. We inputted those absorbance values into the Miller unit formula to calculate enzymatic activity per cell per milliliter.
| Strain Identification Number | Promoter Part Number | RBS Part Number | Relative Strength of Promoter/RBS |
|---|---|---|---|
| R (positive control) | BBa_J23115 | BBa_B0035 | Reference/Reference |
| 1 | BBa_J23113 | BBa_B0031 | Weak/Weak |
| 2 | BBa_J23113 | BBa_B0032 | Weak/Medium |
| 3 | BBa_J23113 | BBa_B0034 | Weak/Strong |
| 4 | BBa_J23106 | BBa_B0031 | Medium/Weak |
| 5 | BBa_J23106 | BBa_B0032 | Medium/Medium |
| 6 | BBa_J23106 | BBa_B0034 | Medium/Strong |
| 7 | BBa_J23119 | BBa_B0031 | Strong/Weak |
| 8 | BBa_J23119 | BBa_B0032 | Strong/Medium |
| 9 | BBa_J23119 | BBa_B0034 | Strong/Strong |
USTC_2009's MEASUREMENT
|
•••••
University of Texas at Austin iGEM 2019 |
UT Austin iGEM 2019: Characterization of metabolic burden of the Anderson SeriesDescriptionThe 2019 UT Austin iGEM team transformed the Anderson Series promoters into our 'burden monitor' DH10B strain of E. coli, which contains a constitutive GFP cassette in the genome of the cell. GFP expression fluctuates depending on the number of ribosomes available. Using this strain, we characterized the relative burden (percent reduction in growth rate) of each Anderson Series part. Our results showed a range of growth rate reductions for each of these parts due to ribosomal reallocation from the genome of the host cell, towards the expression of RFP. Anderson Series parts with strong promoters are depicted with darker red colors and Anderson Series parts with weak promoters are depicted with lighter pink colors to show relative RFP expression. We saw a positive correlation between relative promoter strength and metabolic burden; parts with stronger promoters expressed less GFP and had a lower growth rate than parts with weaker promoters. The regression line for the graph below was constructed by measuring the burden of 5 parts that were created by the 2019 UT Austin iGEM team that each contained an Anderson Series promoter (BBa_J23104 or BBa_J23110), an RBS of varying strength, and a BFP reporter. For more information on characterization of these parts through the burden monitor, visit our team’s wiki page: [1]
Importance of Characterizing BurdenAlthough often we cannot avoid using a specific burdensome part, knowing in advance that it is burdensome, and that it has a high chance of mutating into a non-functional genetic device, can help with troubleshooting and coming up with alternatives. In the specific case of fluorescent protein-expressing devices, Fluorescence-activated cell sorting (FACS) can be used to filter out individual cells that meet a certain fluorescence threshold. This way, the cells expressing lower levels of the fluorescent protein are weeded out of the population. |